What is the product of the reaction and what is the mechanism of the reaction?
CH3-C-(CH3)2-Br + C2H5OH?
1-chloro-1-ethylcyclopentane + CH3OH?
cyclohexane-(C-Br-CH3)-CH3 + H-C=O-OH?
CH3-C-(CH3)2-Cl + a)D2O
b)cyclohexane-OD
CH3-C-(CH3)2-Br + C2H5OH?
1-chloro-1-ethylcyclopentane + CH3OH?
cyclohexane-(C-Br-CH3)-CH3 + H-C=O-OH?
CH3-C-(CH3)2-Cl + a)D2O
b)cyclohexane-OD
1 Answer
All of these involve bulky substrates, so these will all contain some mixture of
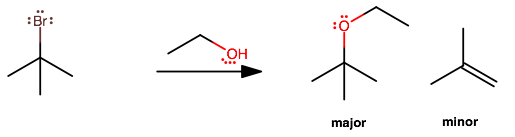
The major product forms from
#S_N1# ; the alkyl bromide is tertiary, so it is bulky/sterically-hindered, and is more likely to lose#"Br"^(-)# as a leaving group before managing to get attacked by a nucleophile (ethanol). The proton on the attached ethanol then dissociates on its own.That is characteristic of a first-order process, where the slow step is the departure of the leaving group and a carbocation intermediate forms (here, a tertiary carbocation).
There is a minor product from
#E1# , but this is very minor unless the temperature is sufficiently high. This would be a#beta# elimination, i.e. the alcohol extracts a proton from the carbon adjacent to#"Br"# and patches up the carbocation intermediate.
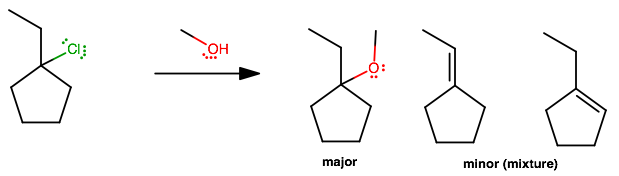
Basically the same idea as in
#(1)# , except...The only difference is a mixture of elimination minor products. Either alkene is equally-substituted (both have one doubly-substituted and one singly-substituted
#sp^2# carbon), so by Zaitsev's rule, either minor product is likely to occur.
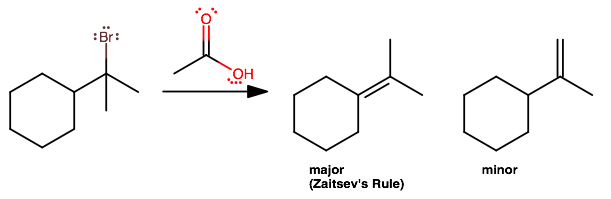
Basically the same idea as in
#(1)# and#(2)# , except now the so-called nucleophile, acetic acid, does not do its job well at all; it's quite bulky, and is better at being a Lewis base (ugh, a base? That'll take gusto, as it's an acid! Gotta wait for it to dissociate as much as possible!).So here,
#E1# is likely, while#S_N1# pretty much won't happen (i.e. some ridiculously bulky ester won't form).By Zaitsev's rule, we expect the more substituted alkene to form, which is the left one (because it has two disubstituted
#sp^2# carbons, compared to the right-hand one).
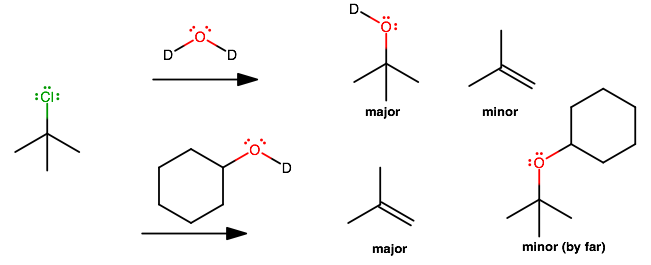
Reaction with deuterated compounds is not that different from the undeuterated forms. It is just easier to tell which
#"H"# is which.
#a)# is a mixture of#S_N1# and#E_1# since the alkyl halide is bulky, as in#(1)# .#E1# at a sufficiently high temperature.
#b)# The difference with respect to#(a)# is that now the reactant added is more bulky. In this case, the major and minor products switch, because substitution would require the approach of a big molecule towards a bulky molecule.Thus, the kinetically-slow monodeuterated cyclohexanol prefers to be a base and perform
#E1# to give the thermodynamically-favored product.

