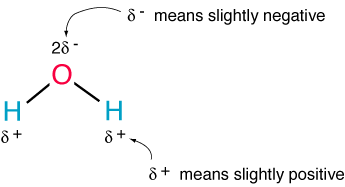What is the property of water that makes it the universal solvent?
1 Answer
It could be its polarity.
Explanation:
Water is often considered the universal solvent, one of the best (if not, the best) in the world at dissolving various substances.
The polarity of water is responsible for many of its properties, one of which is its great ability to dissolve various compounds.
Below is a diagram of the polarity of the water molecule:
When a water-soluble solute is placed in water, the solution process begins immediately.
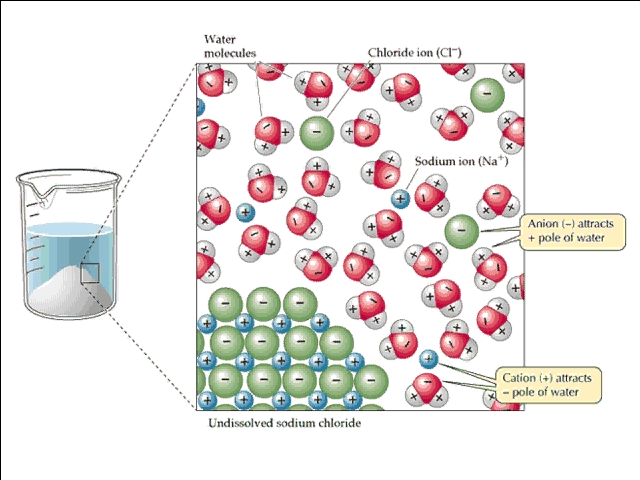
What happens in the case of an ionic compound is the negative end of water molecules (the more electronegative oxygen end) surround the cation, and the positive end of the water molecules (the more electropositive hydrogen end) surround the anion.
The process of dissolving ions in solution is called solvation, and process of the ionic compound splitting into its component ions is called dissociation. Below is an image of the solvation process for sodium chloride: