What is the R/S nomenclature?
1 Answer
Explanation:
It is a fact that a carbon in a tetrahedral array with FOUR different substituents, i.e.
Because such chirality is ubiquitous in biological chemistry, it follows that chemists have had to develop a flexible nomenclature capable of describing chiral carbons and structures.
What you got in the diagram is a so-called Fisher projection.
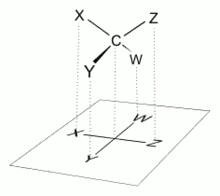
The horizontal
And another picture...

This last perspective means that the methyl group of the given diagram projects into the page, and methyl is the least-ranked substituent. The priority of the other substituents is
And how do you learn this stuff? Make models, and consider your text, and old exam questions. It does not matter how clever, or learned you are, if you cannot vizualize, and assign, AND then REPRESENT the geometry you are a shot bird in these questions.

