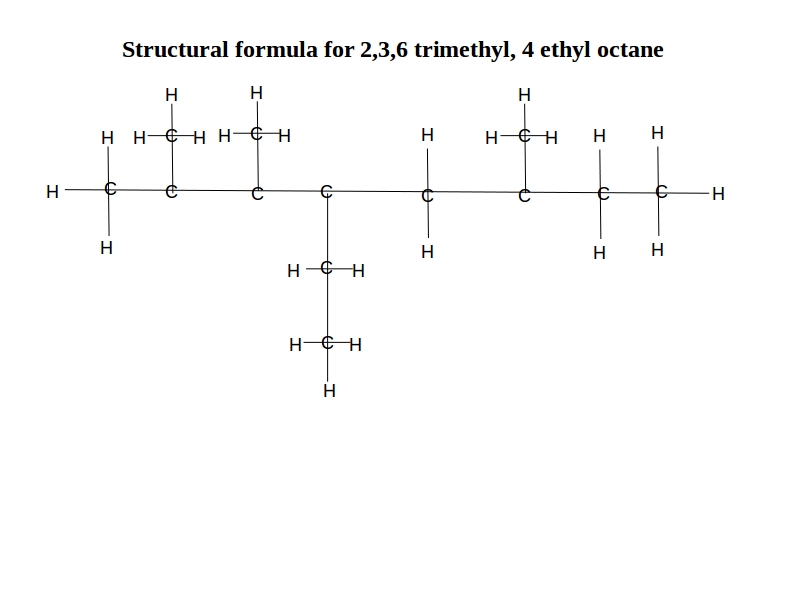
What is the structural formula for 2,3,6 trimethyl, 4 ethyl octane?
1 Answer
Feb 4, 2017
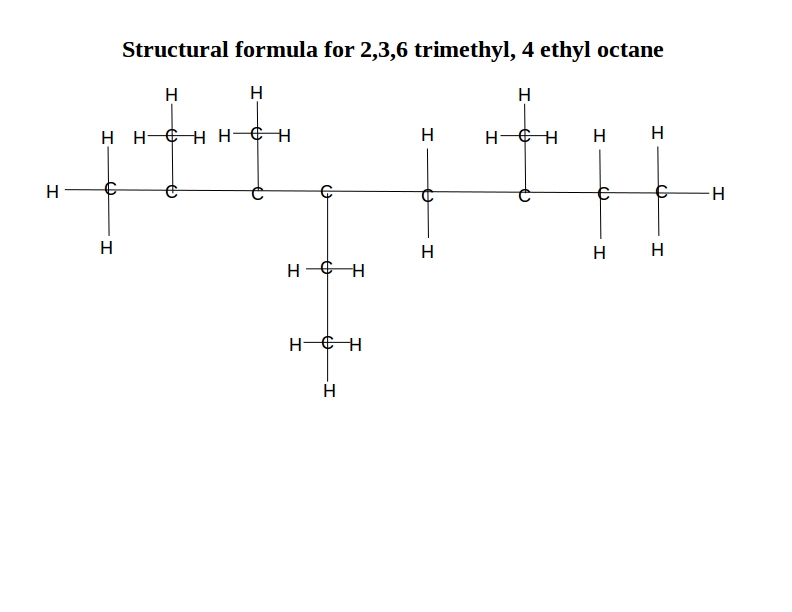
Explanation:
The numbering is the number of the carbon atom on the main “backbone” molecule – octane in this case.
This formula indicates that it has methyl (CH3) groups on the 2nd, 3rd, and 6th carbon, and an ethyl (C2H5) group on the 4th carbon. So, it is a straight 8-carbon chain with the side groups branching off. It looks like this: