What is the structure of L(+) Glucose and L(-) Glucose? What does +, -, and L tell you?
1 Answer
L-glucose is the mirror image of D-glucose.
Explanation:

D-glucose can exist in the straight-chain form (above) and as four different cyclic structures. In solution, it consists of an equilibrium mixture of α-D-glucopyranose

and β-D-glucopyranose.

The "pyranose" part of the name tells us that it is a six-membered ring.
The α form rotates polarized light clockwise (to the right) by +112.2°. The β form rotates polarized light to the right by +18.7°.
The equilibrium mixture is 36% α and 64% β, with a rotation of +52.7°.
The terms "+" and "-" refer to the direction of rotation. A clockwise rotation is "+".
Thus both forms of D-glucose are D-(+)-glucose.
L-glucose is an equilibrium mixture of α-L-glucopyranose

and β-L-glucopyranose.
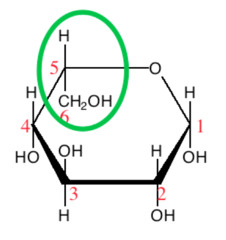
Their specific rotations are -112.2° and -18.7°, respectively.
Thus, both forms of L-glucose are L-(-)-glucose.
There is no such thing as L-(+)-glucose.

