What is the temperature change in 224 g of water upon the absorption of 55 kJ of heat, the specific heat of water is 4.18 J/g °C?
1 Answer
Explanation:
To obtain the change in temperature, let's use the equation below:
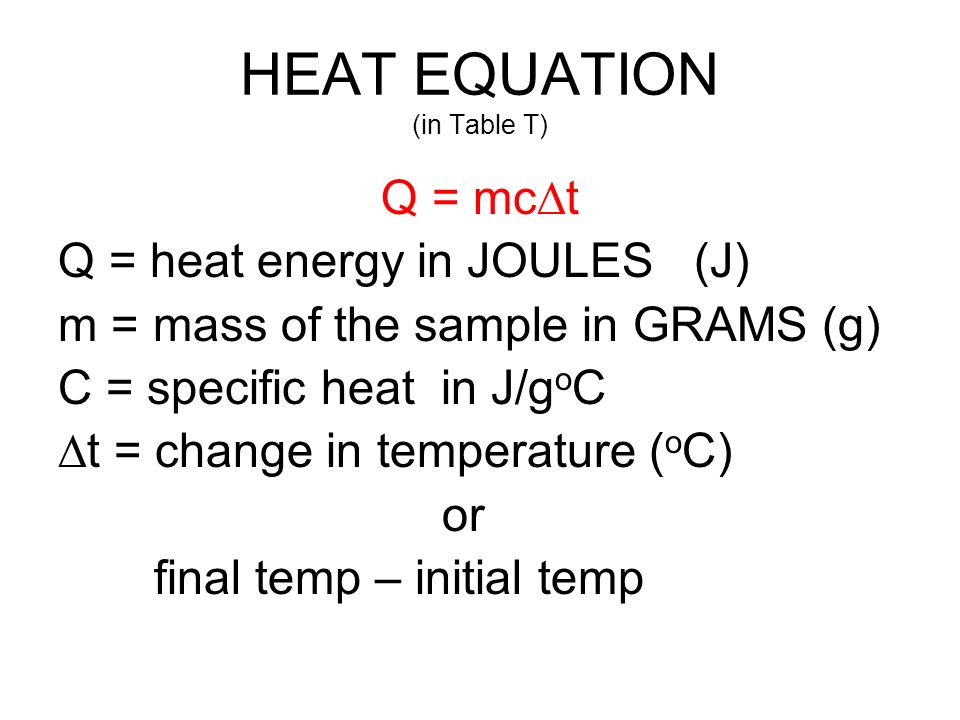 http://slideplayer.com/slide/4758097/
http://slideplayer.com/slide/4758097/
In this question, heat has units of kJ, which does not match up with the units given in the image above. So what we can do first is convert kJ to J by multiplying that value of
 http://converters360.com/energy/kilojoule-to-joule-conversion.htm
http://converters360.com/energy/kilojoule-to-joule-conversion.htm
Here are the variables that we know:
All we have to do is rearrange the equation to solve for
Now, we just plug in the known values:

