What is the total number of orbitals possible at the #n = 4# energy level?
1 Answer
Nov 13, 2017
hmmm...
Back to the Aufbau principle, and the Madelung Rule I think...
Explanation:
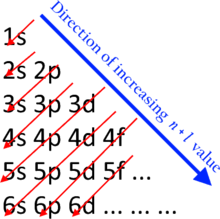
I assume there will be no Level 5 included, so it stops at 4p,,,
Therefore:
In every level, there are:
one
three
five
seven
etc.
Therefore:
1 x
1 x
3x
1 x
3x
1 x
5x
3x
Total: 1+1+3+1+3+1+5+3 = 18 orbitals.....
Image credits: https://en.wikipedia.org/wiki/Aufbau_principle

