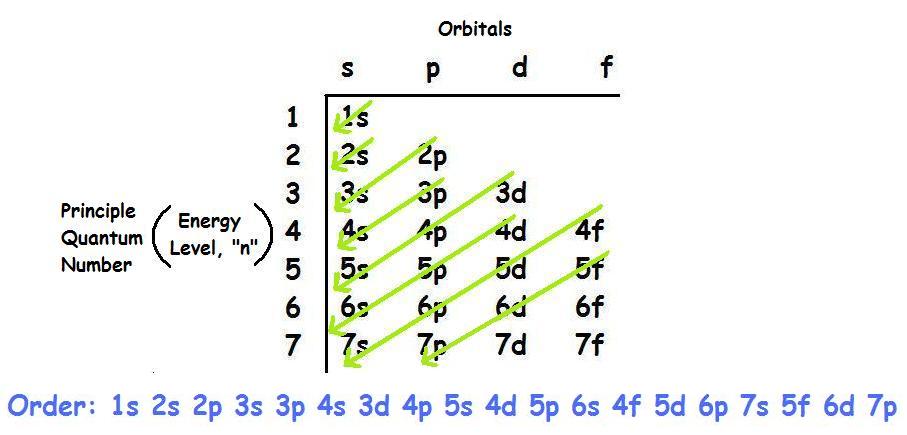
What is the valence electron configuration for phosphorus?
1 Answer
The valence electron configuration for phosphorus is
Phosphorus has an electron configuration of
Phosphorus is found in group 15, the other non-metals on the periodic table.
Phosphorus is in the 3rd energy level, (3rd row) and 3rd column of the 'p' block
The valence electrons are always found in the 's' and 'p' orbitals of the highest energy level of the electron configuration making the valence orbitals 3s and 3p and making the valence configuration
I hope this was helpful.
SMATERTEACHER