What is the volume at STP of 2.66 mol of methane gas?
2 Answers
Explanation:
Because we are at standard temperature and pressure and are given only one set of conditions, we have to use the ideal gas law equation:
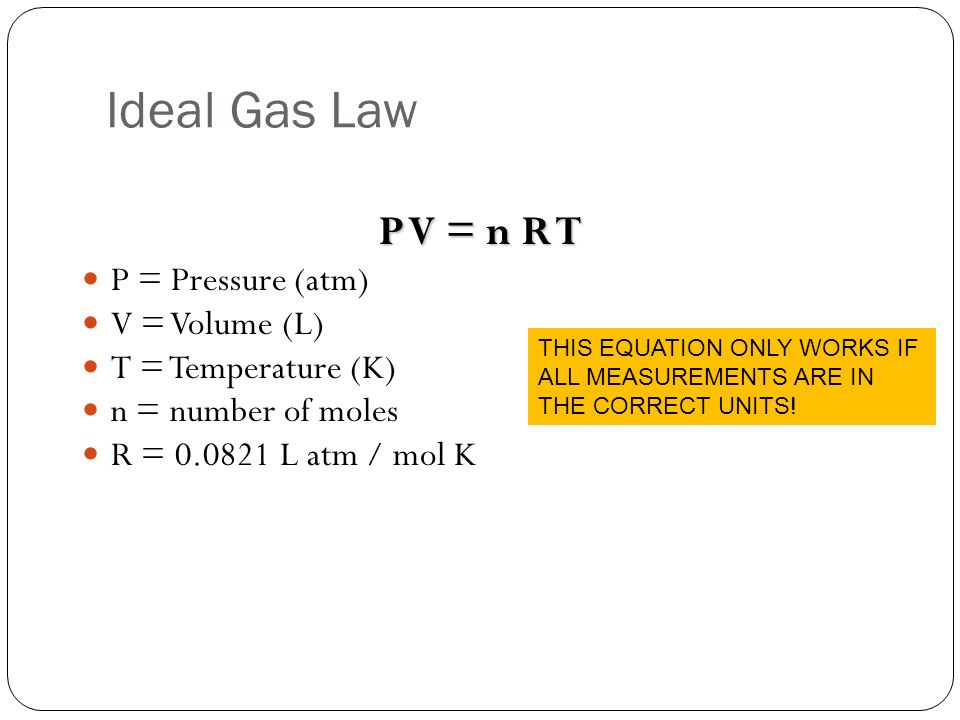
I should mention that the pressure does not always have units of atm, it depends on the units of pressure given in the gas constant.
List your known and unknown variables.
- Number of moles
- Temperature
- Pressure
Volume of
At STP, the temperature is 273K and the pressure is 1 atm.
Let's rearrange the equation to solve for V:
Because the temperature and pressure are given as STP, all that is need is to use the molar volume.
Explanation:
1 mole = 22.4 liters.
so # 2.66 moles / 1.00 mole =
#V/ 22.4#
2.66 x 22.4 = 59.58 liters.

