What is the volume of Carbon dioxide released at SLC per MJ of energy generated?
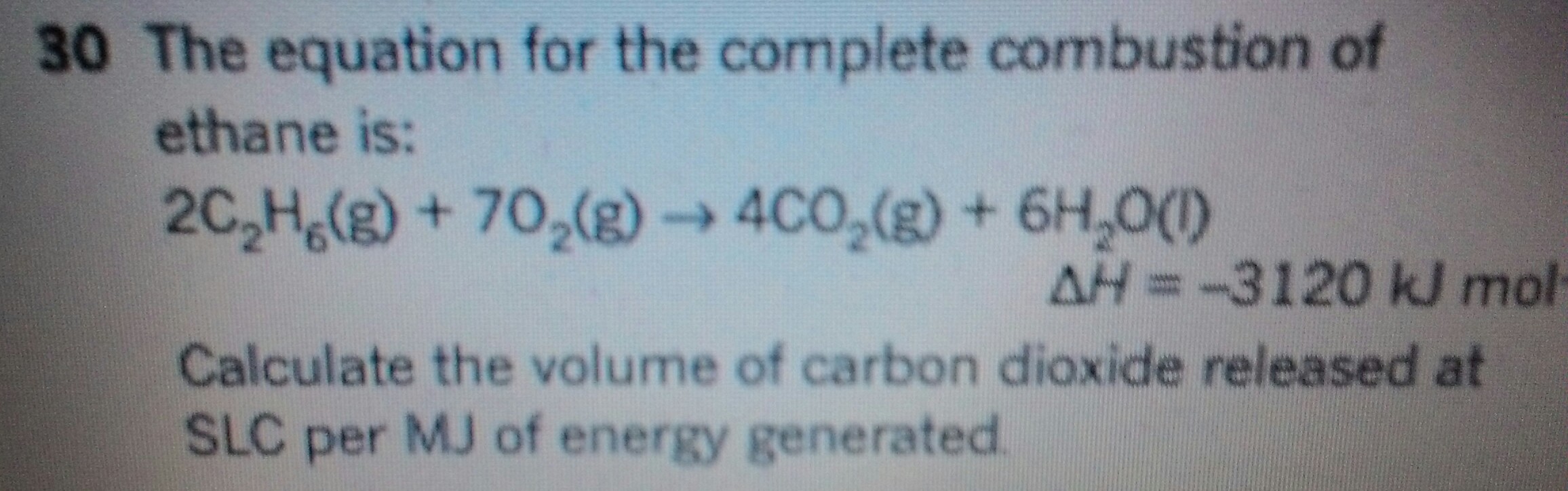
1 Answer
Jan 3, 2018
Approx.
Explanation:
The energy released by combustion of one mole of ethane is
And production of this energy gives rise to TWO moles of carbon dioxide....the which at SLC has a volume of
Now combustion of one mole of ethanol clearly gives rise to 2 moles of carbon dioxide....and so we take the quotient...
i.e.

