What is the volume of the oxygen gas required for this combustion reaction? (Question-image in the description box below)
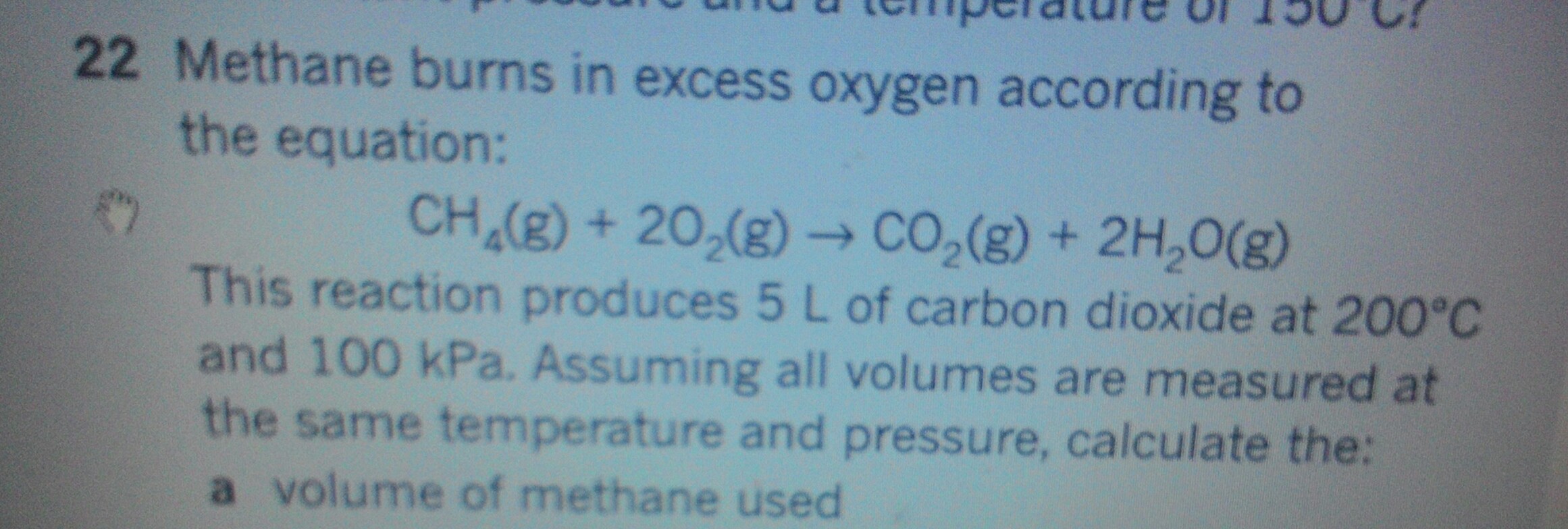
1 Answer
The SAME volume of methane was used. i.e.
Explanation:
You gots the combustion reaction...
We get a
The stoichiometric equation (which you provided) is KEY. Of course, we might say, that the water product condensed, but still one volume of carbon dioxide results from combustion of one volume of methane....
With me?
Had we thermodynamic data, then we could also assess the heat produced by the combustion reaction. But remember all I am doing is assessing the given stoichiometric equation.
Happy?

