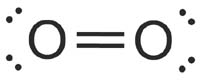What kind of bond would the electron dot formula for #O_2# show?
1 Answer
May 25, 2017
Explanation:
Both atoms of oxygen in the
Each oxygen atom looks like this:
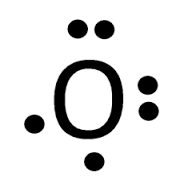
They each have two lone electrons which need to be paired up. So, the two lone electrons from one oxygen atom bond with the two lone electrons from the other oxygen atom, forming a double bond.
The Electron Dot Diagram for