What property of elements visibly show periodic trends when their values are graphed?
1 Answer
Explanation:
Clearly there are many periodic properties in the periodic table, but a salient one is effective nuclear charge. A property I find helpful in understanding other properties. In addition there is ionization energy, nuclear radius, electronegativity and electron affinity, inter alia.
Effective nuclear charge is the nuclear charge experienced by valence electrons after accounting for electron-electron repulsion forces and screening by core electrons.
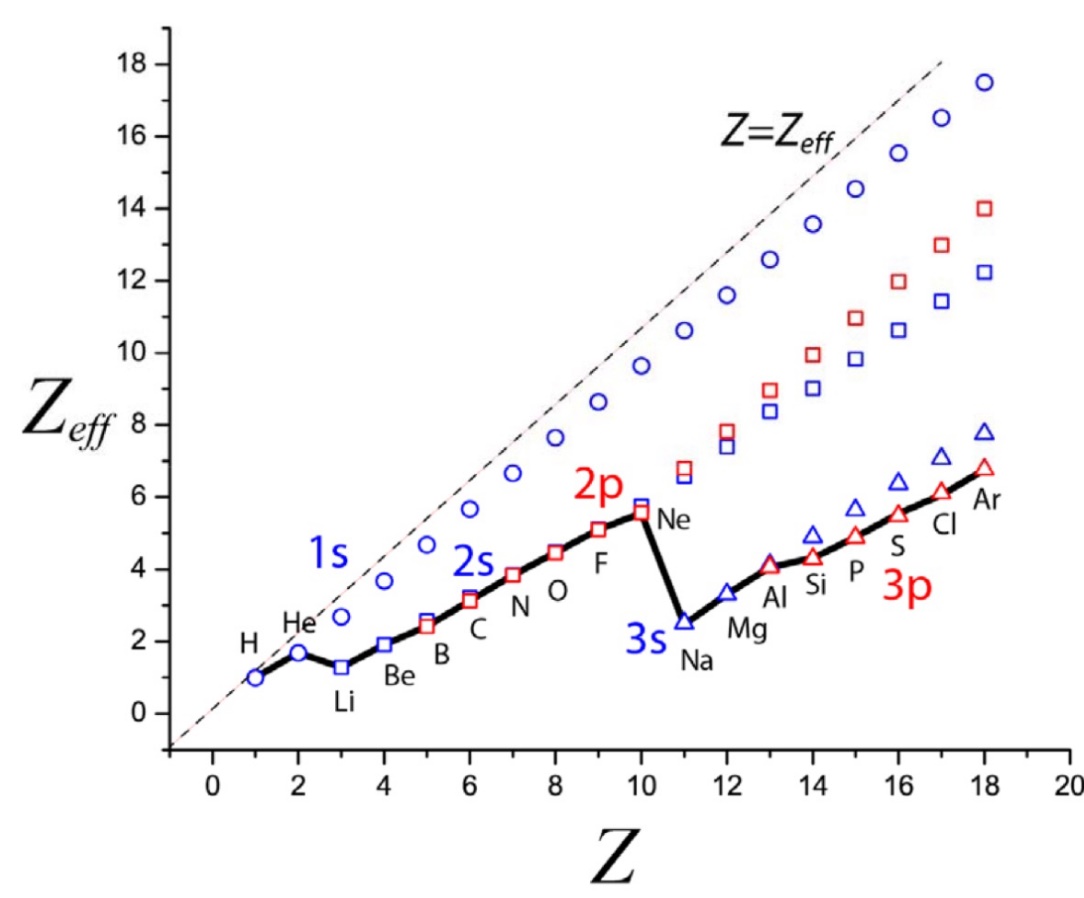
Exploring this graph, we see where is says [Ne] to [Na] a big drop once the 2p subshell and consequently the n=2 shell is filled with electrons. This more diffuse cloud, and in addition increased size of the atom means the valence electron of Na is far less "attracted" to the nucleus than Ne's are.

