What stereoisomers are obtained from hydroboration–oxidation of cis -2-butene?
1 Answer
Feb 2, 2015
The product is a racemic mixture of (
If the BH₃ attacks from the top of the alkene
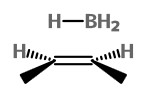
we get the borane
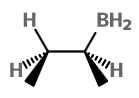
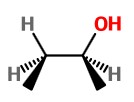
The product on oxidation with H₂O₂ is (
If the BH₃ attacks from the bottom of the alkene
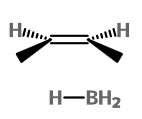
we get the borane
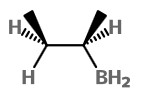
The product on oxidation with H₂O₂ is (
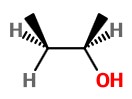
There is an equal chance of attack from top or bottom, so we get a 50:50 mixture of the two stereoisomers.

