What substances conduct electricity by the movement of ions?
3 Answers
Explanation:
Ionic substances conduct electricity by flow of ions; solids do not conduct.
The ions must be dissolved in a solvent in order to flow and conduct electricity.
These are called electrolytes.
Explanation:
Some substances in aqueous solution can conduct electricity due to the movement of dissolved ions throughout the solution.
Substances that dissociate into component ions when placed in aqueous solution are called electrolytes.
Which substances are electrolytes?
Basically any soluble ionic compound, acids and bases are electrolytes, because they have capacity to dissolve into ions. Molecules like
There are two classes of electrolytes:
-
Strong electrolytes
-
Weak electrolytes
Strong electrolytes are those in which the compound dissociates almost completely into its component ions.
Weak electrolytes are those in which the compound ionizes only a small amount in solution.
Here is a visual representation of electricity conduction through aqueous solution:
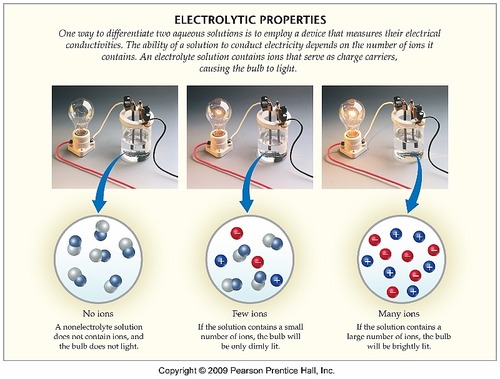
Can you classify the three examples in this image as a strong electrolyte, weak electrolyte, or nonelectrolyte?
Practically all ionic compounds are strong electrolytes, no matter how soluble they are (the amount that does dissolve does so nearly completely).
Acids and bases that are strong electrolytes are called strong acids and bases. Acids and bases that are weak electrolytes are called weak acids and bases.
Here is a list of the strong acids and bases:
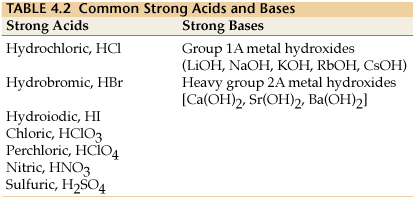
Know that if an acid or base is not on this list, it is most likely a weak acid or base and dissociates into ions only partly.
Conduction of electricity by the movement of ions is a characteristic of metal salts when dissolved into water which can quickly dissociate the molecules of the solute into ions in solvation.
Explanation:
When a salt is formed by the reaction of a metal element with one of the gaseous halogens, the resulting compound can be readily broken down into free radical ions in a solution of water. The molecular angle of the water molecules overpower the weaker molecular binding forces in the salt, and the molecules break up into negative anions and positive cations.
There is a quick look here:
http://www.bbc.co.uk/schools/gcsebitesize/science/add_edexcel/periodic_table/groupsrev4.shtml
But there is more. One of the best electric insulators on earth is air. But during a lightening storm, tremendous potential differences in the atmosphere can become powerful enough to ionize the local air, and result in flashes of various types of lightening generation.
The process is described here:
https://hps.org/publicinformation/ate/q9399.html
See photos of lightened-up areas here:
http://www.nationalgeographic.com/environment/natural-disasters/lightning/

