What volume do one mol and three mol of gas occupy at STP?
1 Answer
Standard Temperature and Pressure conditions imply a temperature of 273.15 K and a pressure of 1 atm.
When these conditons are met, 1 mole of any ideal gas occupies exactly 22.4 L. Keep in mind that ideal gas particles are assumed to have no volume of their own, that's why, for example, at STP 1 mole of helium gas will occupy the same volume as, say, 1 mole of chlorine gas.
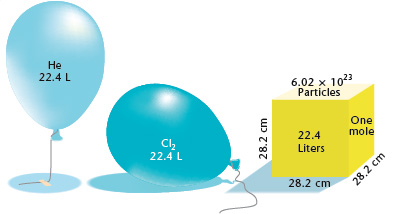
However, as you can see in the picture, the two gases will have different masses because of the difference in their molar mass. The balloon filled with helium will weigh less than the one filled with chlorine, despite the fact that they occupy the same volume.
So, if 1 mole occupies 22.4 L, the imediate conclusion is that a bigger number of moles will occupy more than 22.4 L, and a smaller number of moles will occupy less than 22.4 L.
In your case, 3 moles of gas will occupy 3 times more volume than 1 mole of gas.
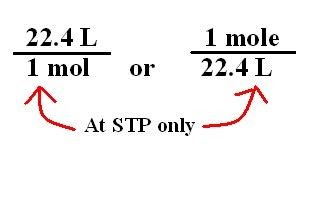
Likewise, 0.5 moles will occupy half the volume 1 mole occupies

