What volume (in liters) does 2.895 moles of oxygen occupy at STP?
1 Answer
Jul 2, 2016
At STP, 2.895 moles of
Explanation:
Since we are at STP and are given only one set of conditions, we have to use the ideal gas law equation:
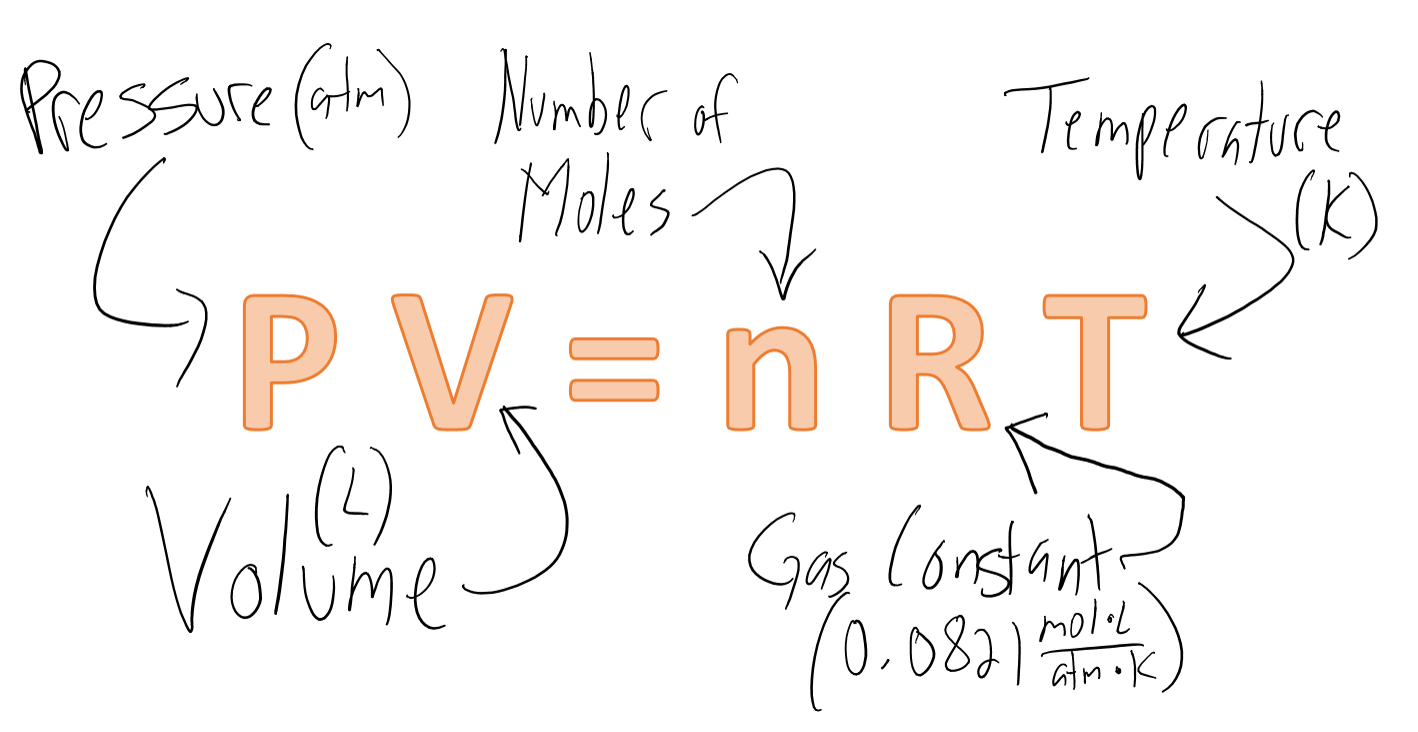 chemistryhungergames.com
chemistryhungergames.com
I should mention that the pressure does not always have units of atm, it depends on the units of pressure given in the gas constant.
List your known and unknown variables. Our only unknown is the volume of
At STP, the temperature is 273K and the pressure is 1 atm.
Now we have to rearrange the equation to solve for V:

