What volume of a 2.5 M stock solution of acetic acid is required to prepare 100.0 milliliters of a .50 M acetic acid solution?
1 Answer
Aug 22, 2016
Explanation:
Whenever you are dealing with a dilution, (going from a higher concentration to a lower concentration), you can use the equation below:
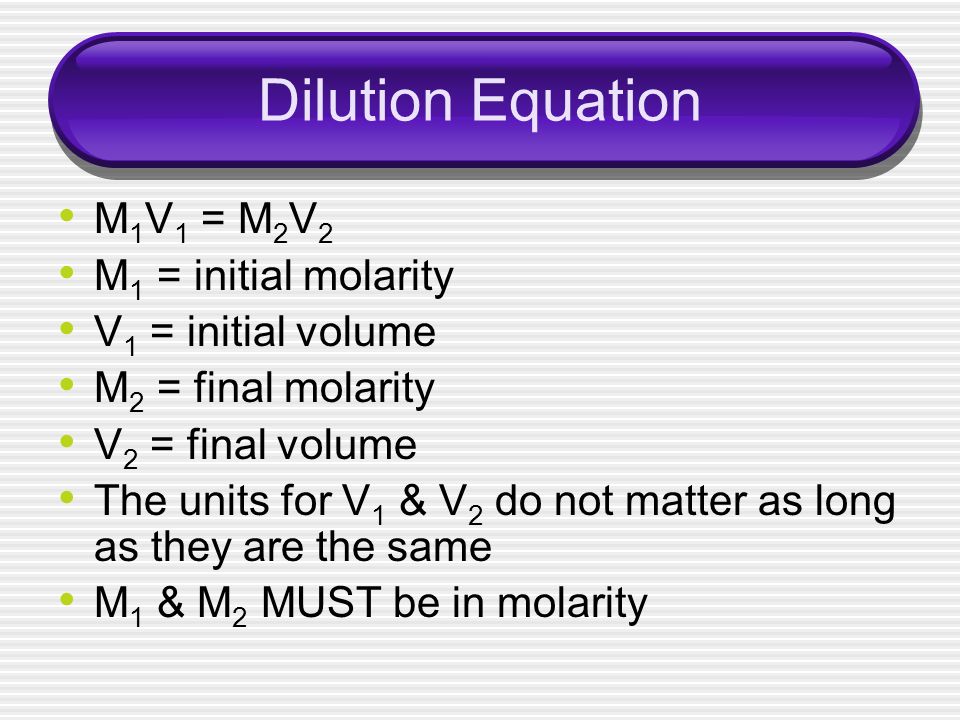
Let's determine our known and unknown variables:
- Initial Volume
Next, we rearrange the equation to solve for
Plug in the known values and solve for your unknown:
Therefore,

