What will be the major product obtained on dehydration of propan-1,2-diol ?
I am not being able to understand which one among the 2 lone pairs (of 2 oxygen atoms) is going to attack the proton.
I am not being able to understand which one among the 2 lone pairs (of 2 oxygen atoms) is going to attack the proton.
1 Answer
Jan 24, 2018
I predict the major product to be propanal.
Explanation:
The sulfuric acid protonates both oxygen atoms.
Protonation at
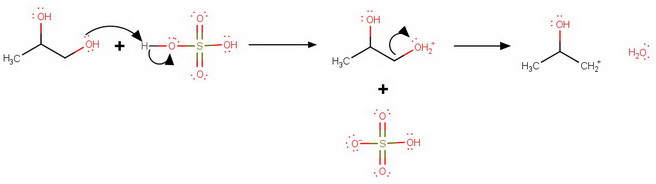 C1 Protonation
C1 Protonation
However, loss of water from
Protonation at
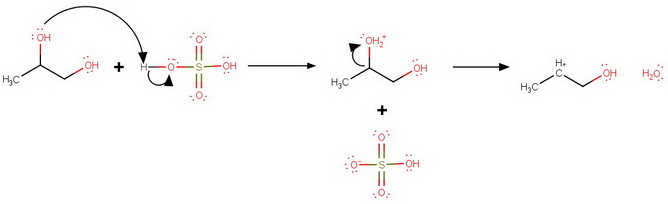 C2 protonation
C2 protonation
Loss of water from
Loss of an α-hydrogen
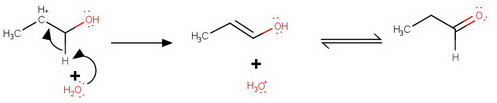 Deprotonation
Deprotonation
The cation loses a proton preferentially from

