What will be the product when C6H6Cl6 is heated with alc. KOH? Please explain the mechanim too.
1 Answer
The reaction will occur if all the
the reaction will not occur if the
Explanation:
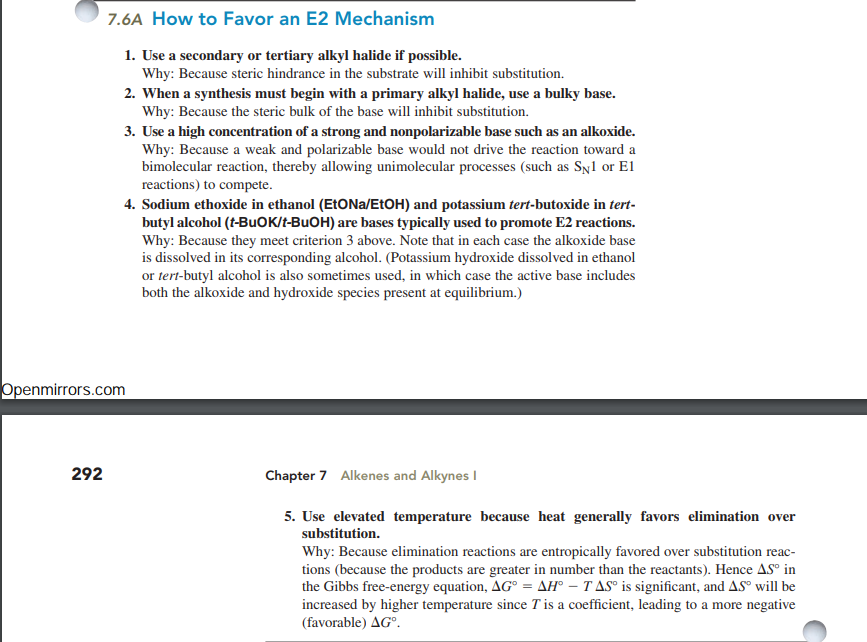
NOTE :-
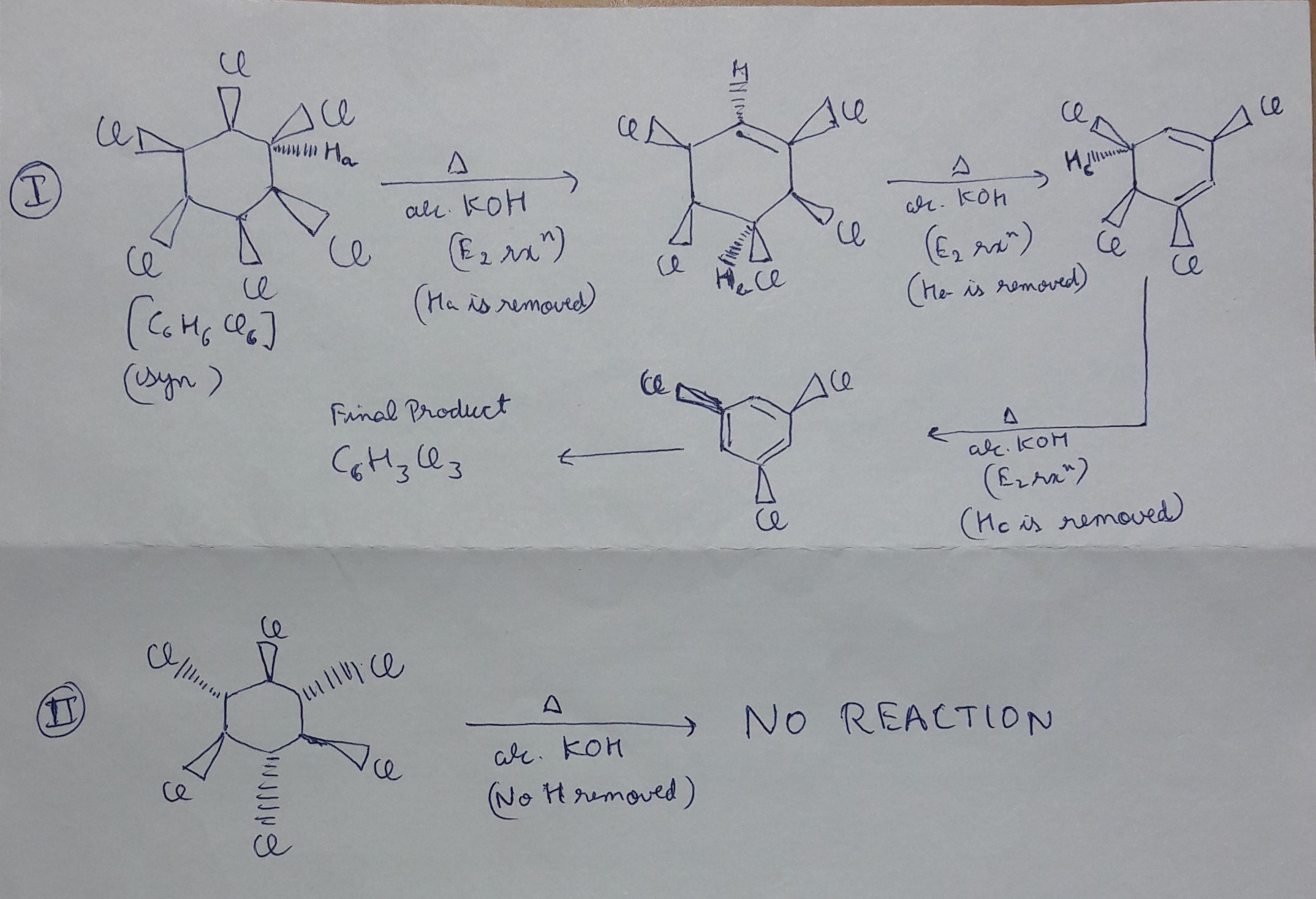
The reaction of Alkyl halide
General

The reaction will occur if all the
the reaction will not occur if the
NOTE :-

The reaction of Alkyl halide
General

