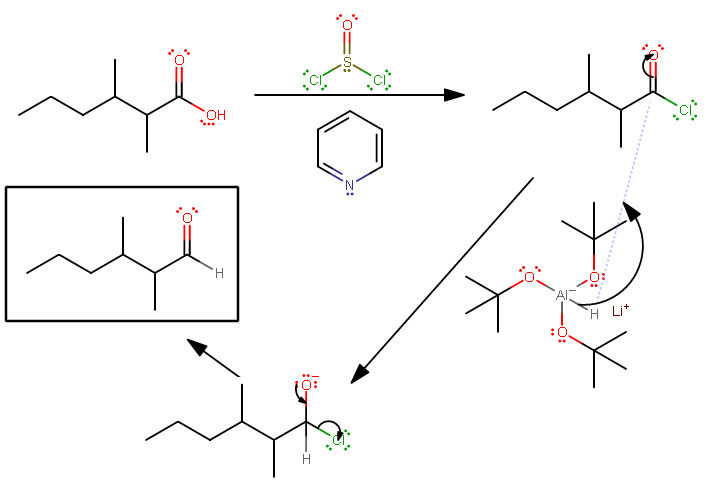
When 2,3-dimethylhexanoic acid is treated with SOCl2/pyridine, followed by LiAl [OC(CH3)3]3H, what would be the product???? Thank you
1 Answer
The first mechanism is as follows:
Pyridine is the solvent. Its protonated form's pKa is higher than that of
Then the big aluminum compound (lithium tri-tert-butoxyaluminum hydride, Ch. 20, Organic Chemistry, Paula Yurkanis Bruice) with the electron-donating groups allows the single hydrogen to act as a hydride, and you get a reduction to an aldehyde, rather than all the way to an alcohol.
Lithium tri-tert-butoxyaluminum hydride is nice to use because it's so bulky and it has only one hydride. Diisobutylaluminum hydride (DIBALH) can be used as a similarly useful alternative.
Both single-hydride aluminum compounds should be used in cold temperatures (e.g.
So, the overall reaction would be written as: