When an electron jumps from a high energy state to a lower state, what form does the emitted energy take?
1 Answer
Jul 7, 2016
A typical radiative process in which an electron jumps from a higher-energy state to a lower-energy state started with kinetic energy, which is converted into light energy that is emitted.
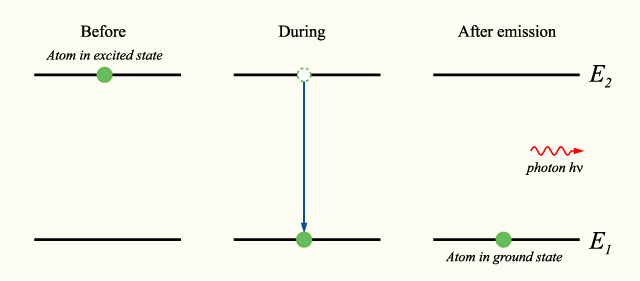
Note that energy is not necessarily emitted in any relaxation process.
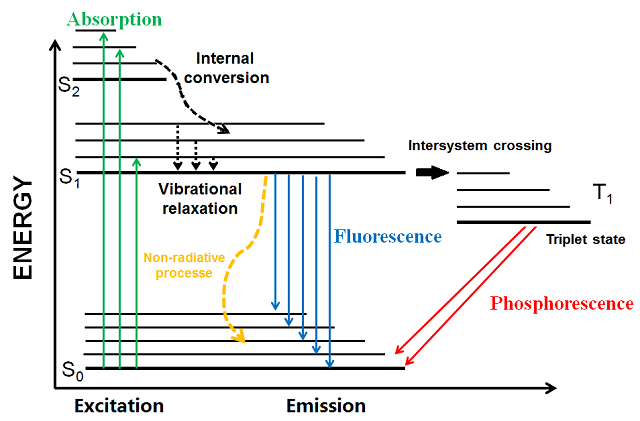
You may have processes like vibrational relaxation, intersystem crossing, and internal conversion, which are non-radiative (usually marked by dashed arrows), i.e. they do not radiate energy after they occur.
On the other hand, processes like fluorescence and phosphorescence may emit light energy, i.e. they are radiative (marked by solid arrows).

