When are the bonds of ions both covalent and ionic?
Are all polyatomic ions both covalent and ionic? I heard mixed things
Are all polyatomic ions both covalent and ionic? I heard mixed things
1 Answer
Sep 30, 2017
I think you've confused yourself... Nothing can have covalent bonds that are also themselves ionic bonds.
Some polyatomic ions have covalent bonds within them. But clearly, they are ions, and so they interact ionically with other ions.
Not all polyatomic ions have covalent bonds...
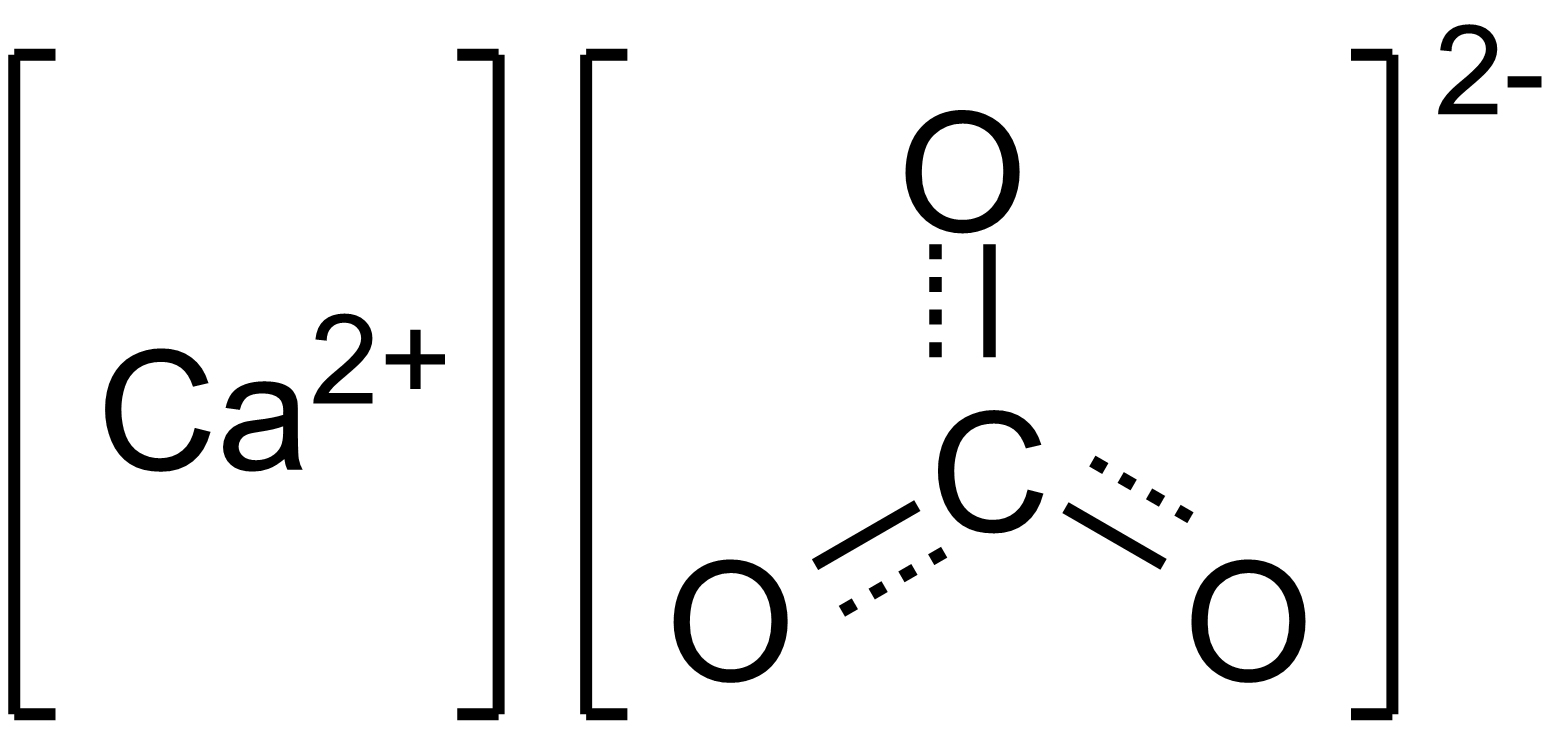
Carbonate,
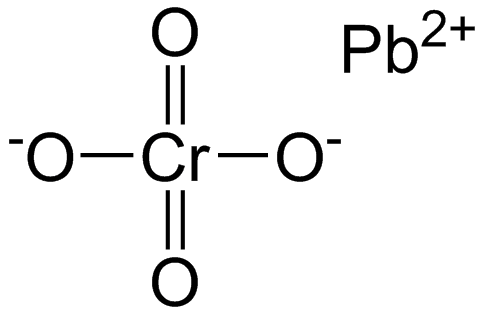
Chromate,
All polyatomic ions interact ionically with metals. Not all polyatomic ions are molecular.

