When does vaporization take place?
1 Answer
Jan 15, 2016
Vaporization takes place at all temperatures.
Explanation:
Vaporization is the conversion of a liquid or a solid into a gas.
At any given temperature, the molecules of a liquid (or a solid) have an average kinetic energy that is proportional to the temperature.
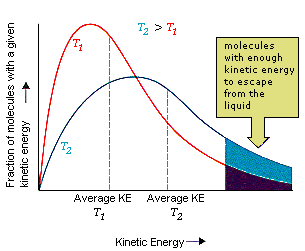
There is a distribution of kinetic energies, so some of the molecules at the surface have enough energy to escape into the gas phase.
(from en.wikipedia.org)
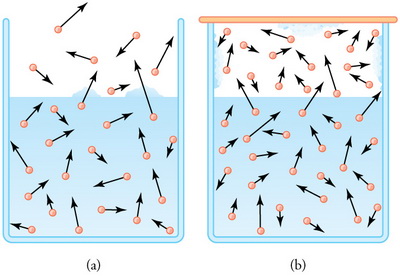
The liquid is evaporating.
The equilibrium pressure exerted by the vapour in a closed container is called the vapour pressure.
The vapour pressure decreases as the temperature decreases but, theoretically at least, there will always be some molecules with enough energy to escape into the vapour state.

