When iron(II) sulfate dissolves in water, does iron(II) form a complex ion with water surrounding it?
1 Answer
Yes. All iron sulfate compounds complex with water until all the binding sites are filled up.
In this case, you get an octahedral complex that can be written as
where the oxygens on the waters point towards the iron.
A common name for it is Ferrohexahydrite. The scientific name for this would be either hexaaquairon(II) sulfate or iron(II) sulfate hexahydrate.
You can call
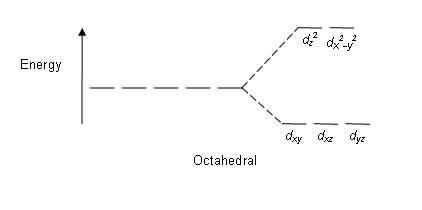
The waters are known as the ligands in this metal-ligand complex. Water is a relatively weak-field ligand, meaning that it generates only small repulsive forces that do not cause much splitting of the
 https://upload.wikimedia.org/
https://upload.wikimedia.org/
That means that the energy levels of the

