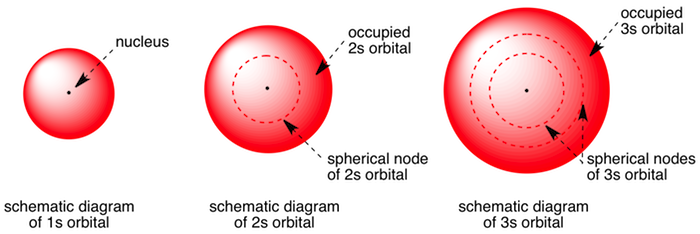
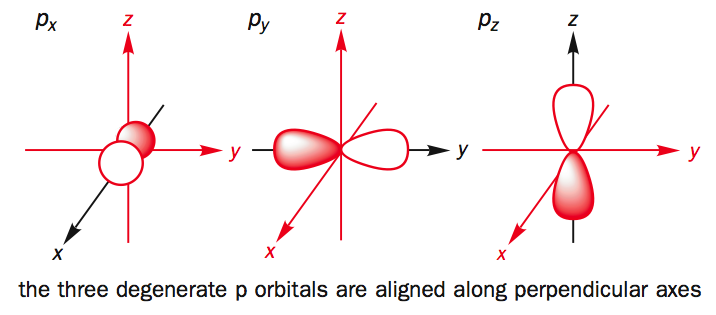
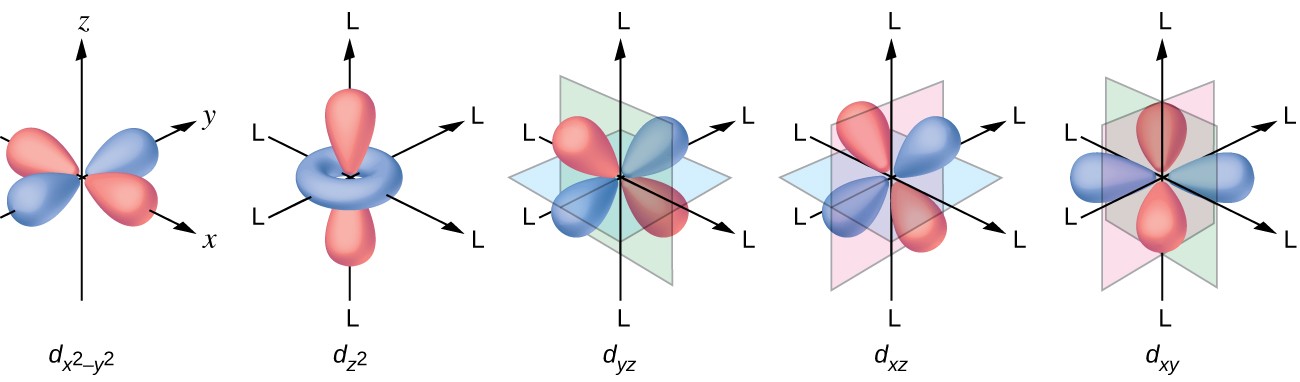
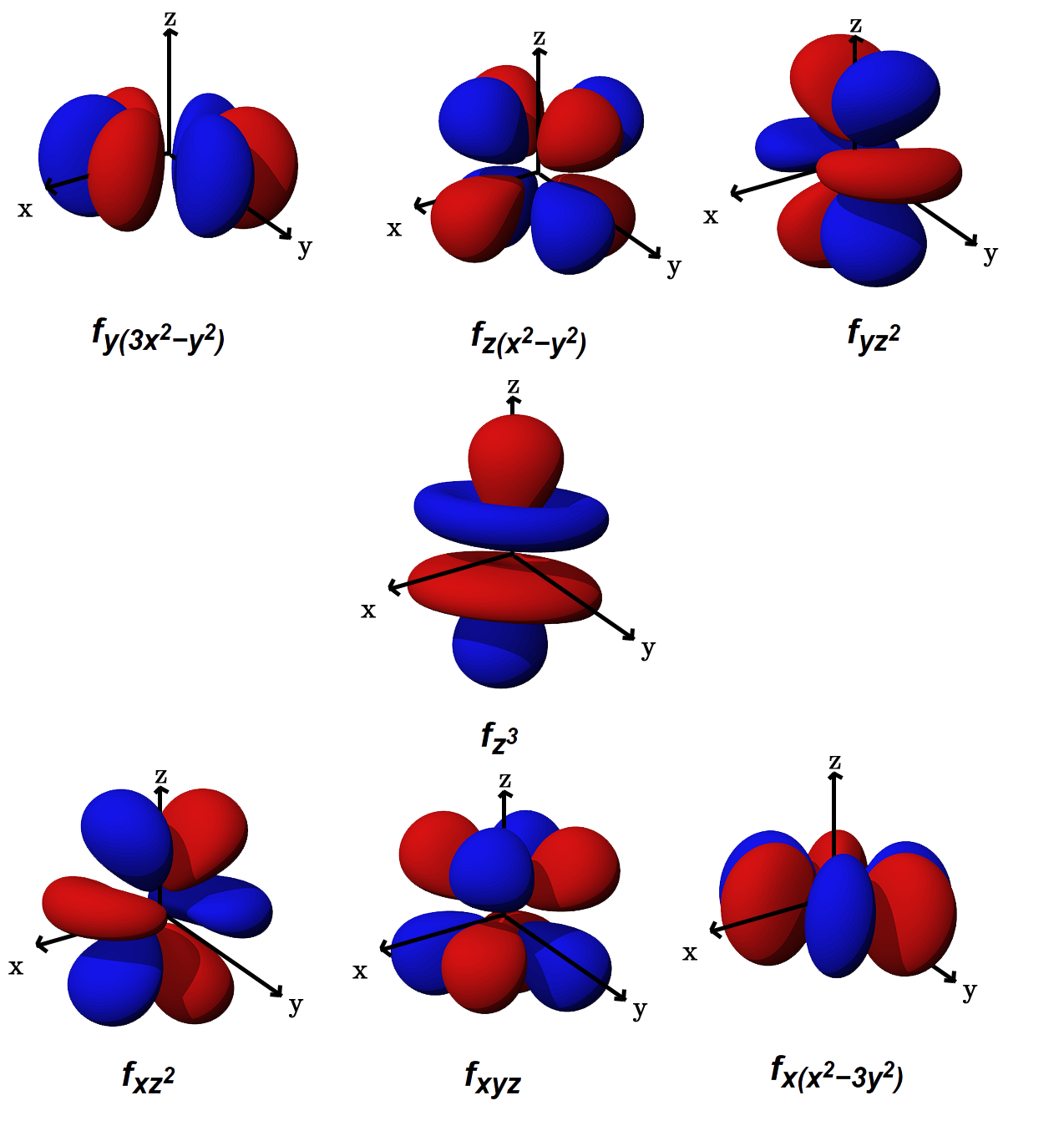
Which are the orbitals(s,p,d,f) have center of symmetry?
1 Answer
Jun 3, 2018
Orbitals with
Orbitals with a center of symmetry have even (gerade) symmetry with respect to inversion, i.e. if the coordinates are inverted so that
Orbitals without such a thing have odd (ungerade) symmetry with respect to inversion.
#s# orbitals are gerade
#p# orbitals are ungerade
#d# orbitals are gerade
#f# orbitals are ungerade