Which compound has the highest melting point? State the reason!
1 Answer
The first compound has the highest melting point.
Explanation:
The two significant factors affecting the melting points of fatty acids are
- Chain length
- Degree of unsaturation
Chain length
The longer the chain length, the higher the melting point.
Let's number the compounds 1 to 5 from top to bottom.
The structure of acid 5 (octanoic acid) is
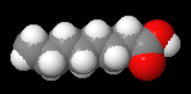 Caprylic
Caprylic
The structure of acid 1 (hexadecanoic acid) is
 Palmitic
Palmitic
The molecules of the saturated fatty acids have a rod-like structure.
Thus, they can readily form a crystal in which they line up to make a stable crystalline lattice.
The longer molecules have stronger London dispersion forces because of their length, so they have higher melting points.
Degree of unsaturation
The more double bonds in the chain, the lower the melting point.
Acid 2, 3, and 4 are 16-carbon acids with *one, two, and three double bonds.
I don't have pictures of these, but I have similar ones for 18-carbon acids.
Octadecanoic acid is a saturated fatty acid with melting point 69 °C.
 Stearic
Stearic
The double bonds in unsaturated fatty acids all have the
Thus, oleic acid (octadec-9-enoic acid) has the structure below.
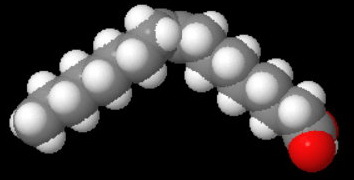 Oleic
Oleic
The cis-double bond introduces a kink, so the molecules cannot pack as neatly into a crystal lattice.
The melting point of oleic acid is 14 °C.
A linoleic acid (octadeca-9,12-dienoic acid) molecule has two kinks.
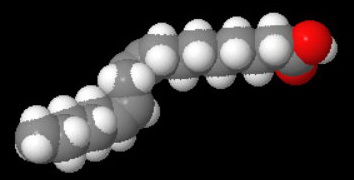 Linoleic
Linoleic
The melting point is -8 °C.
A linolenic acid (octadeca-9,12,15-trienoic acid) has three kinks and a still lower melting point.
 Linolenic
Linolenic

