Which compound reacts with acidic dichromate (Cr2O72- / H+) to give the product shown?
The answer is C, but I don't understand that why it is not D.
The answer is C, but I don't understand that why it is not D.
2 Answers
It's not the same compund
Explanation:
Yeah, they are isomers ( they have the same molecular formula ) but:
oxidation of C) leads to 2-methyl propanoic acid ( the one on the right in the picture )
oxidation of D) leads to butyric acid
Those are also catene isomers
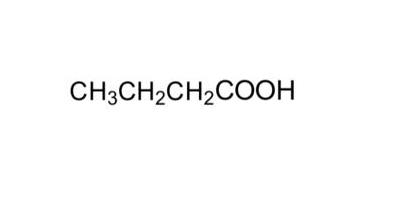
! it's not the same thing
Compound C reacts to give the product shown.
Explanation:
Compound A
Acidified dichromate does not oxidize tert-butyl alcohol under normal conditions.
Compound B
Secondary alcohols form ketones on oxidation.
This is not the observed product.
Compound C
Primary alcohols form carboxylic acids on oxidation.
This is the observed product.
Compound D
This is not the observed product.


