Which compound would you expect to have the smallest heat of hydrogenation:3,4-dimethyl-2-hexene, 2,3-dimethyl-2-hexene, or 4,5-dimethyl-2-hexene?
1 Answer
Dec 12, 2014
The compound with the smallest heat of hydration is 2,3-dimethyl-2-hexene.
The structure of 3,4-dimethyl-2-hexene (A) is CH₃CH₂CH(CH₃)C(CH₃)=CHCH₃.
The structure of 2,3-dimethyl-2-hexene (B) is CH₃CH₂CH₂C(CH₃)=C(CH₃)₂.
The structure of 4,5-dimethyl-2-hexene (C) is CH₃CH(CH₃)CH(CH₃)CH=CHCH₃.
If you draw the structures, you find that A is a trisubstituted alkene, B is tetrasubstituted, and C is disubstituted.
The order of stability of substituted alkenes is
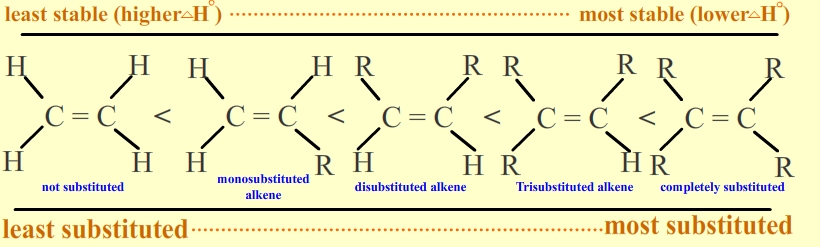
So the most substituted alkene (B) is at the lowest energy level.
The dimethylhexane hydrogenation products should all be at about the same energy level.
The heat of hydrogenation diagram would be like that below.

So B has the lowest heat of hydrogenation.

