Which conjugate base is more stabilized?
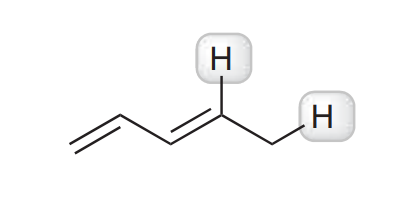
I think this is carbon from top because he can spread charge over two more carbons so is delocalized but answer for that is carbon from right. Am i wrong or answer is not good?
I think this is carbon from top because he can spread charge over two more carbons so is delocalized but answer for that is carbon from right. Am i wrong or answer is not good?
1 Answer
Apr 15, 2018
Resonance will do nothing if we deprotonate the proton you're proposing. You have the right idea about delocalization, though.
Let's give those two carbon's a charge:

Hence,

