We would expect #"BF"_3# to be stronger, because #"F"# is more electronegative than #"Cl"#.
Chemists explain this unexpected result by an electronic argument and a steric argument.
The electronic argument — backbonding
The boron atom in #"BF"_3# is #sp^2# hybridized, with a vacant #2p# orbital.
The #"F"# atoms can also be #sp^2# hybridised, with lone pairs in their #2p# orbitals.
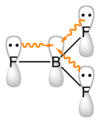
These #"F"# orbitals can overlap with the orbital on #"B"#, thereby increasing the electron density on the boron atom and making it less acidic.
This effect is called backbonding, because electron density is leaving the more electronegative atom.
In #"BCl"_3#, the #3p# orbitals on #"Cl"# are bigger than the #2p# orbital on #"B"#, so orbital overlap is less efficient, and backbonding is less important.
Hence, the greater backbonding in #"BF"_3# makes it a weaker Lewis acid.
The steric argument — ligand close-packing (LCP)
The LCP model is based on the observation that the #"X"# atoms (ligands) in #"AX"_n# systems are always the same distance from each other.
For example, the distance between the #"F"# atoms in #"BF"_3# and #"BF"_4^-# is 226 pm, despite the longer #"B-F"# distance in the tetrahedral structure.
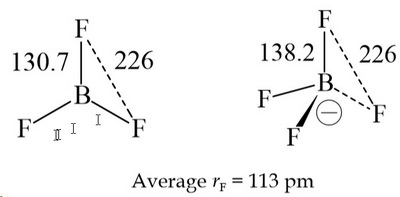
(from alpha.chem.umb.edu)
It is as if the #"F"# atoms are closest-packed (like in a crystal), with the #"F"# atoms having a ligand radius of 113 pm.
When the #"BF"_3# forms a Lewis complex, the #"F"# atoms remain close-packed, but the #"B-F"# bonds must become longer in the new tetrahedral geometry.
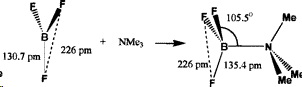
(from http://pubs.acs.org/doi/abs/10.1021/ic990713m)
It takes more energy to lengthen the short, strong #"B-F"# bonds than the longer, weaker #"B-Cl"# bonds.
Hence #"BF"_3# is a weaker Lewis acid than #"BCl"_3#.




