Which is more stable vanadium or chromium? And why?
1 Answer
It is not a physically reasonable comparison to make. They are different elements, and each of them are as stable as that element can be in their respective ground states.
Now, if you meant which element is more prone to participating in chemical reactions, it would usually be vanadium, as it has lower ionization energies for comparable oxidation states (up to
If we were to compare their ionization energies, we could see that the atom with the lower ionization energy has a greater tendency to lose the
Using data from NIST, I get:
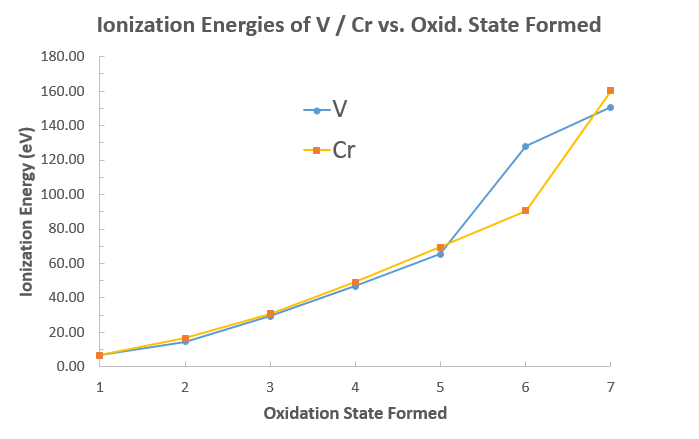
However,

