Which molecule would have a Lewis Structure that is an exception to the octet rule?
1 Answer
They all are because they all contain an atom which will not have eight valence electrons.
Explanation:
Look to see how many electrons the central atom ends up with. If the central atom does not have eight valence electrons it is an exception to the octet rule.
Members of the boron family will be exceptions to the octet rule because they typically form three bonds and end with 6 valence electrons. See the discussion in the video of
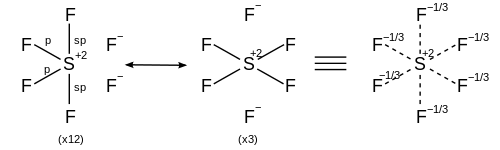
NO is a radical (has one unpaired electron) so it will also be an exception to the octet rule since having an unpaired electron makes it impossible to draw a Lewis structure in which both the N and the O will have eight valence electrons.
Hope this helps!

