Which molecules will undergo haloform reaction?
a) CH3COCH3
b) acetophenone
c) CH3CH2CHO
d) CH3COOH
e) CH3Ctriple bondN
a) CH3COCH3
b) acetophenone
c) CH3CH2CHO
d) CH3COOH
e) CH3Ctriple bondN
1 Answer
Only those capable of forming an enolate, as that is what acts as a nucleophile in this reaction. Typically, that would be if they are methyl ketones.
So, acetone and acetophenone (
Also:
- propionaldehyde would not work because it is more likely to be an electrophile than a Bronsted acid to the
#"OH"^(-)# . - acetic acid would rather be a Bronsted acid at the carboxyl
#"OH"# , but you want it to do so at the#alpha# proton instead... - methyl nitrile is probably not reactive enough to do this; nitrogen is not as electronegative as oxygen, so the nitrile carbon is not as electrophilic as in ketones.
The haloform reaction is an enolate reaction.
[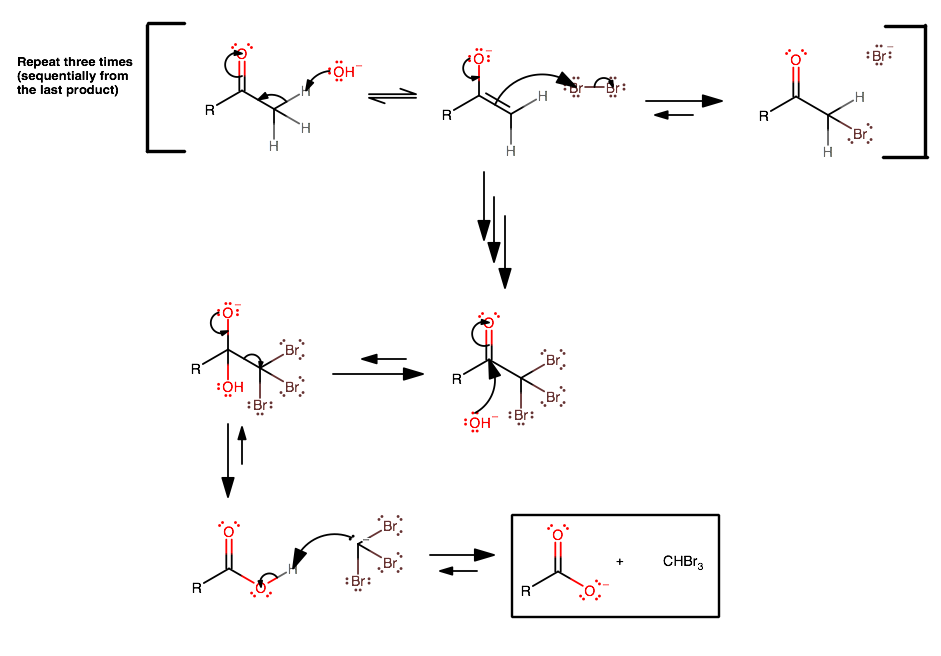
(click to zoom in.)
- A strong base (usually just
#"NaOH"# ) comes in to deprotonate the#alpha# -carbon, forming the enolate. - This is followed by nucleophilic attack upon a diatomic halogen molecule (repeat steps 1 and 2 two more times until the
#alpha# -carbon is saturated with halogen atoms). -
Strong base comes in (e.g.
#"NaOH"# ) as a nucleophile this time (due to the loss of all#alpha# -protons).However, this step is thermodynamically difficult in base, since the three halides make the carbonyl carbon more electronegative via inductive electron withdrawal, lessening its susceptibility to nucleophilic attack.
-
Once the tetrahedral intermediate does form, the resultant
#""^(-)"CX"_3# is a good (enough) leaving group since there are three electron-withdrawing groups on the carbon. One may need to push the equilibrium with additional#"OH"^(-)# . - The mechanism finishes with a deprotonation of the resultant carboxylic acid to form the haloform and the carboxylate.

