Which of the entities have higher atomic radius? a) Ca, Ca2+ b) S, S2-. Please explain it?
2 Answers
For a) the neutral Ca atom has a larger radius than the
This is because the loss of electrons reduces the shielding effect between the electrons and the nucleus, so the attraction of the nucleus for the electrons is greater, reducing the radius.
For b)
When an atom gains one or more electrons, forming an anion, the added electron(s) increase the shielding effect between the outermost electrons and the atomic nucleus. This results in a reduced attraction between the electrons and the nucleus, and the radius increases.
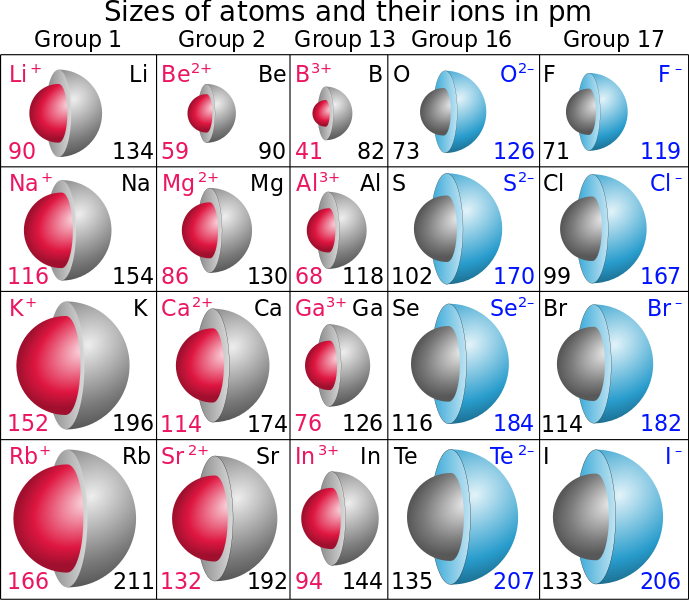
The neutral atoms are gray, the cations are red, and the anions are blue.
I meant which had higher atomic radius for each pair not comparing a pair to another pair



