Which of the following are paramagnetic?
#O_2^(2-) ,O_2^- ,O_2#
1 Answer
May 31, 2018
Well
Explanation:
...the ion contains NO UNPAIRED electrons. And
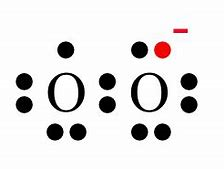
...contains ONE UNPAIRED electron....this beast is PARAMAGNETIC.
And, surprisingly, dioxygen gas,
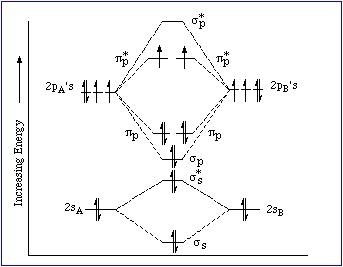
And so

