Which of the following compounds will undergo an Sn2 reaction most readily: # (CH_3)_3C CH_2I# or #(CH_3)_2CHI#?
1 Answer
To make this question less complicated, it is helpful to draw the structures of both compounds as shown in the image below:
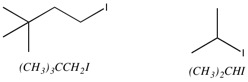
Take a look at the carbon atom bound directly to the iodine.
For
An incoming nucleophile will often react with whatever electrophile it can reach most easily. In technical terms, a secondary halide is more sterically hindered than a primary halide, so
We should therefore expect the isopropyl iodide to have the slower reaction rate.
In
You would therefore expect this compound to have the fastest
The bulky t-butyl group prevents backside attack by the nucleophile.

The steric hindrance is so effective that isopropyl iodide reacts almost 3000 times as fast as neopentyl iodide.


