Which of the following has/have a strong covalent bond? (A) Cl-F (B)F-F (C)C-Cl. (D)C-F?
1 Answer
Mar 17, 2018
Well, all of them....
Explanation:
The strongest covalent bond is PROBABLY between
As a chemist, as a physical scientist, you should consult the data...
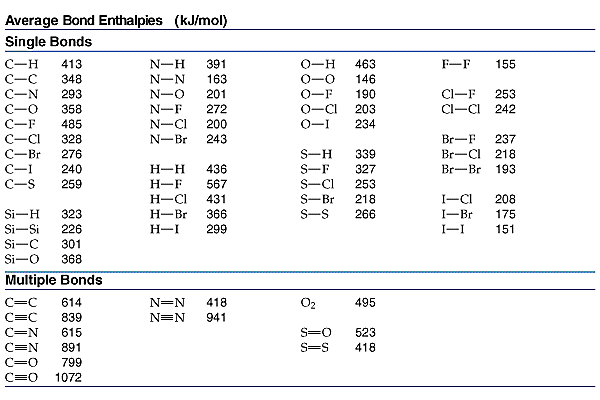
The weakness of the

