Which of the following has highest bond energy?
#S-S# #O-O# #Se-Se# #Te-Te#
#S-S# #O-O# #Se-Se# #Te-Te#
1 Answer
I would assume
Explanation:
Why? We ASSUME that we interrogate the strength of an
From first principles we would expect that the smaller the atom, the BETTER the overlap between electrons, and the SHORTER the
But we must interrogate data as physical scientists...
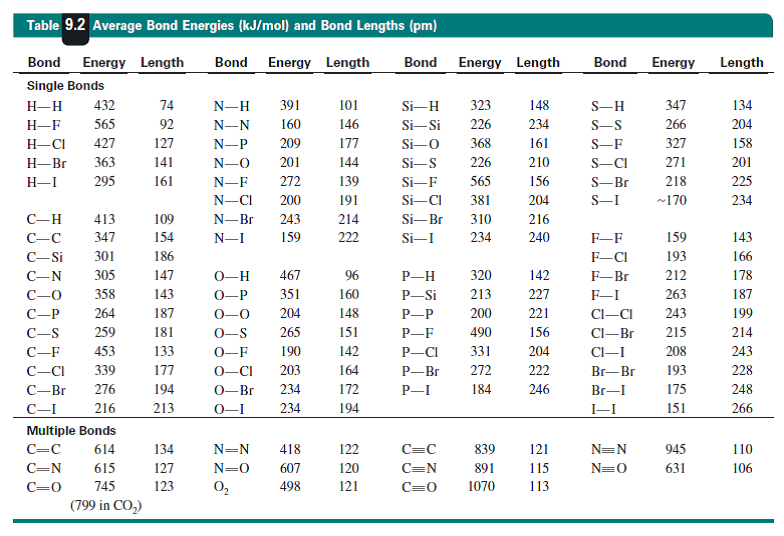
The data are quoted in
Do the measured bond energies, and the bond distances, support my argument? Bugger me, they do not. The homonuclear tellurium and selenium bonds are not reported. However the peroxo bond is
We assume that the lone-pair/lone-pair repulsion that occurs for the peroxide link, and these lone-pairs are small, and should interact destructively, weakens and lengthens the
I hope the quoted data are right. But on this basis the question is quite unfair, and certainly you should request feedback from your lecturer/tutor on this question.

