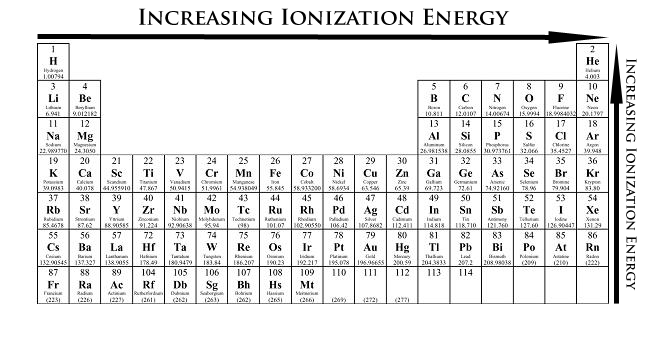
Which of the following has highest first ionization energy? 1)Sc 2)K
1 Answer
1)
Explanation:
The first ionization energy refers to the energy it takes to remove one electron from an atom.
It mainly depends on the electrostatic attraction between the positive protons in the nucleus and negative electrons—if protons and electrons are more attracted to each other, then the first ionization energy would be higher, and vice versa.
Here's the periodic table, with the positions of
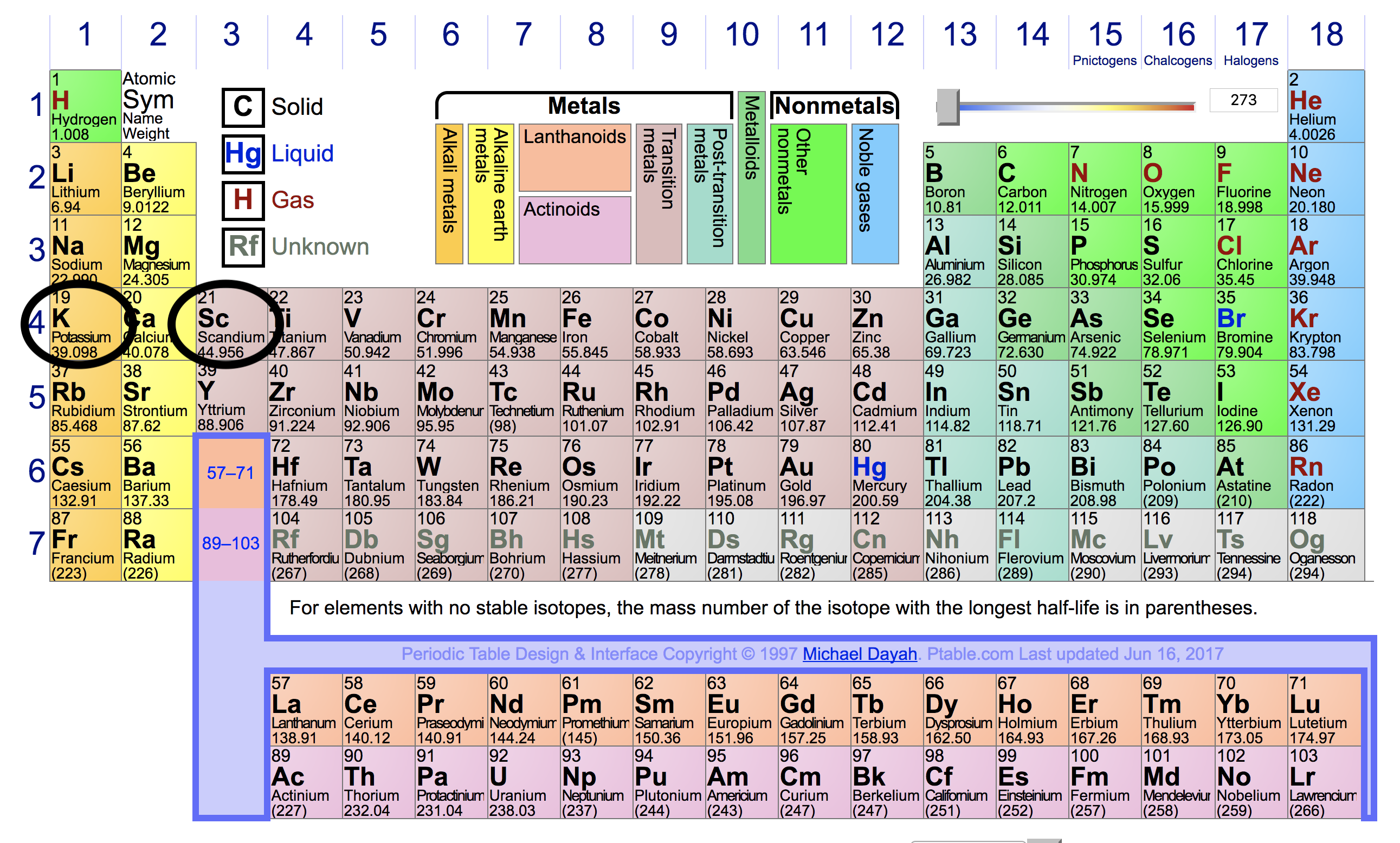
From this, we can see that
However, there is a key difference between the two: a neutral
So, the magnitude of attraction between the positively charged protons and negatively charged electrons is greater for
This fits with the trend of ionization energy increasing as we move right, across a period, too!