Which of the following is most stable carbocation and why?
2 Answers
I would think it is
But since we are spreading out positive charge, I could see how
What if the positive charge in
Before I make any conclusions for these structural questions, I draw resonance structures. Which one stabilizes (through delocalization) the positive charge best? (Or does it?)
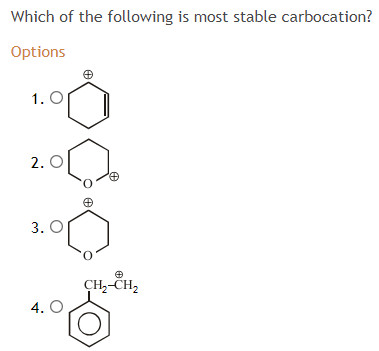
- In this case we find that
bb((2)) delocalizes the positive charge over more atoms than(1) , and the more spread out the positive charge it is, the less stable the molecular cation becomes.
The partial positive lands on the oxygen, which is an electronegative atom... it prefers to be partially negative. However, this only spreads out positive charge over two atoms, so that is OK.
- Structures
(3) and(4) have no resonance structures that do anything for the positive charge.
Here is my contribution to the discussion.
Explanation:
I would start my argument at the other end.
4. is the least stable because it is a primary carbocation with no resonance stabilization.
3. is a little more stable because it is a secondary carbocation, but it has no resonance stabilization.
We must choose between 1. and 2. Both of these are stabilized by resonance.
 Resonance
Resonance
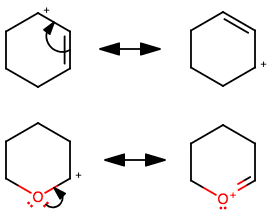
Which is more stable: an allylic carbocation or a carbocation with an adjacent alkoxy group?
The only difference I see between the two carbocations is this:
Resonance participation of the
This may be the reason why structure 2. is more stable.
Confirmatory evidence
We can compare the substituent effects on
An adjacent


