Which of the following processes shows a decrease in entropy of the system?
A) 2NO (g) + #O_2# (g) #\rarr2NO_2# (g)
B) #COCl_2# (g) #rarr# CO(g) + #Cl_2# (g)
C) #CH_3OH (l)\rarr# CO(g) + #2H_2# (g)
D) #NaClO_3# (s) #\rarrNa^+# (aq)+#ClO_3^-# (aq)
E) None of the above will show a decrease in entropy.
A) 2NO (g) +
B)
C)
D)
E) None of the above will show a decrease in entropy.
3 Answers
Process A) shows a decrease in entropy.
Explanation:
The entropy of a system increases whenever its particles have more freedom of motion.
Thus, the entropy increases whenever you have more moles of gaseous products than of reactants and whenever you have more product particles in solution than you have of reactant particles.
Conversely, entropy decreases when you have the opposite situations.
A)
You have 3 mol of gas on the left and 2 mol on the right, so entropy is decreasing.
This is the correct answer.
B)
You have 1 mol of gas on the left and 2 mol of gas on the right, so entropy is increasing.
This answer is incorrect.
C)
This has no moles of gas on the left and 3 mol of gas on the right, so entropy is increasing.
This answer is incorrect.
D)
There are more particles in solution on the right than on the left, so entropy is increasing.
This answer is incorrect.
(A)
Explanation:
A) decrease; less moles of gas in product
B) [ Not sure, but it doesn't seem to have changed aside from splitting... Maybe increase? There are more moles in the product... ]
C) increase; liquid to gas (wow, it went straight past solid... that's a lot of entropy isn't it?)
D) increase; solid to aqueous (gas/liquid distributed in solution)
Here's what I got.
Explanation:
The general idea here is that entropy increases as disorder and randomness increase.
Similarly, entropy decreases as disorder and randomness decrease.
Now, randomness and disorder increase as a substance goes from solid to liquid, and finally to gas. On the other hand, randomness and disorder decrease as a substance goes from gas to liquid, and finally to solid.
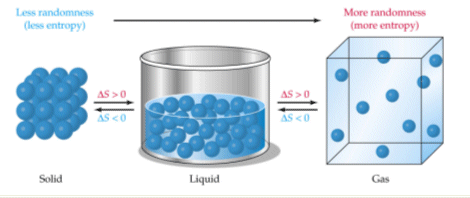
So right from the start, you know that any reaction that has a gas as a reactant and a liquid as a product, for example, will result in a decrease in entropy.
In your case, you have
#"CH"_ 3"OH"_ ((l)) -> "CO"_ ((g)) + 2"H"_ (2(g))#
A liquid is being converted to two gases, so entropy will increase.
#"NaClO"_ (3(s)) -> "Na"_ ((aq))^(+) + "ClO"_ (3(aq))^(-)#
A solid is being dissolved to produce solvated ions, so in general, you can say that entropy will increase. This is not a very good example because there are solids that can be dissolved in water to a decrease in entropy.
I'm not going to go into why that is the case, just keep in mind that it is possible.
Now, the first two reactions involve gaseous reactants and gaseous products. In such cases, look at the total number of moles of gas present on the two sides of the chemical equation.
When it comes to reactions that involve gases, you will have
#"more moles of gas on the reactants' side " -> " decrease in entropy"# #"more moles of gas on the products' side " -> " increase in entropy"#
Notice that this reaction
#2"NO"_ ((g)) + "O"_ (2(g)) -> 2"NO"_ (2(g))#
has a total of
This means that this reaction will show a decrease in entropy.



