Which of the following transitions would result in the absorption of a photon with the longest wavelength?

1 Answer
The absorption with longest wavelength is
Explanation:
The relation between energy
#color(blue)(bar(ul(|color(white)(a/a)E =(hc)/λcolor(white)(a/a)|)))" "#
where
We can rearrange this formula to get
#λ = (hc)/E#
This shows that the wavelength is inversely proportional to the energy: the smaller the amount of energy absorbed, the longer the wavelength.
So, we look for the transition that involves the smallest energy.
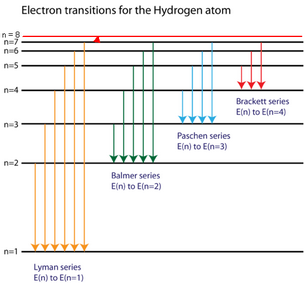
(Adapted from Chemistry LibreTexts)
We see from the energy level diagram that the energy levels get closer together as
This, the smallest energy and the longest wavelength is associated with the
I tried to mark it with an arrow in the diagram, but the lines are so close together that all you can see is the red triangle of the arrowhead.

