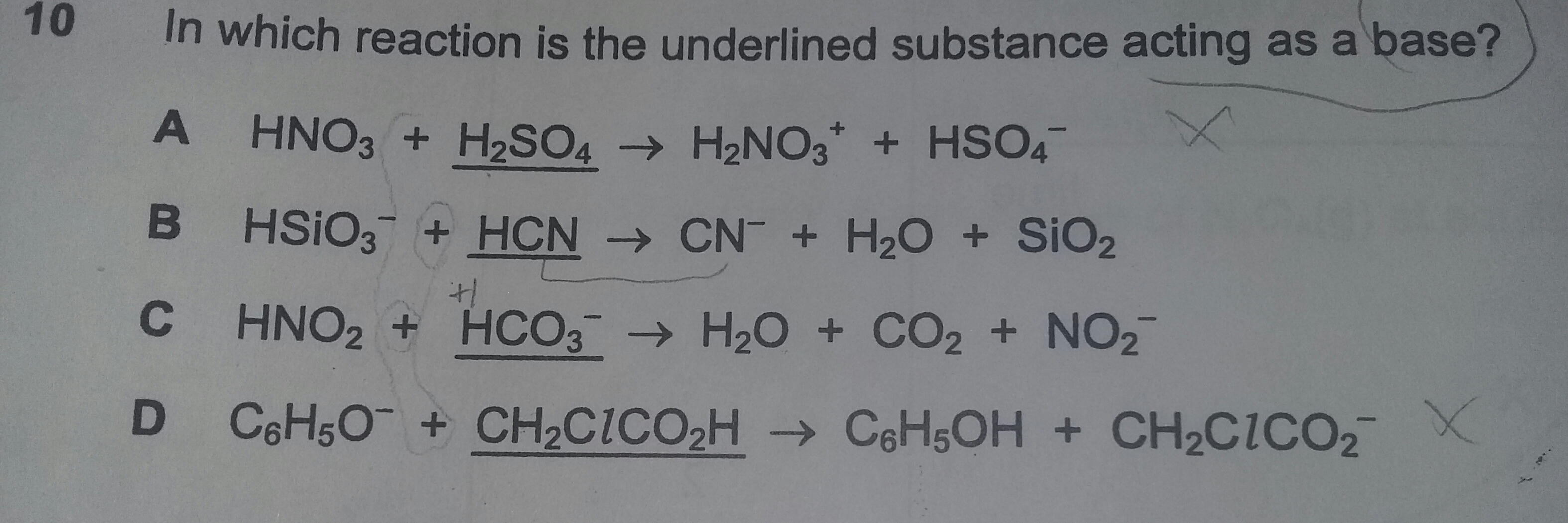
Which of the underlined substance is acting as a base?
1 Answer
Jul 19, 2018
Is it not
Explanation:
A base is a proton acceptor; an acid is a proton donor.
For
For
For
But for
Another way we could represent carbonic acid is as...

