Looks like #D# is chiral to me, and it would have a chiral center on the tertiary carbon external to the bottom-left ring.
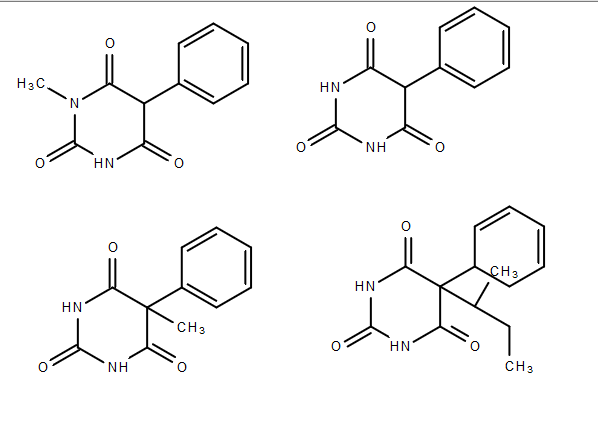
Let the molecules be labeled #A,B,C,D# from left to right (first row = #A,B#, second row = #C,D#).
Well, the tertiary carbon would be a first candidate, if not for the plane of symmetry around it in #B#:
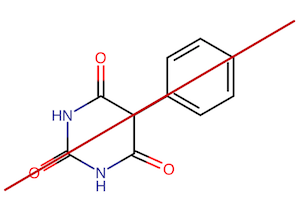
This is due to the identical carbonyls and #"N"-"H"# groups on the left and right side of the benzene ring. So, we can disregard that carbon, because with a plane of symmetry, it can never be chiral. Given the plane of symmetry in the entire molecule, #B# is achiral.
Since we can disregard the tertiary carbon, it follows that #A# and #C# are also not chiral (the only difference with respect to #B# is #"H" harr "CH"_3#).
Further examination of #A# shows two identical carbonyls on either side of the #"N"-"CH"_3#, so the nitrogen there is also not a chiral center (not to mention, it only has three electron groups).
So, that only leaves #D# being chiral, due to the tertiary carbon external to the ring, surrounded by:
- #"CH"_3#
- #"CH"_2-"CH"_3#
- #"H"#
- The carbon we called tertiary earlier, on the bottom-left ring.
Due to having four different groups around it, #bbD# has one chiral center and is a chiral molecule overall.