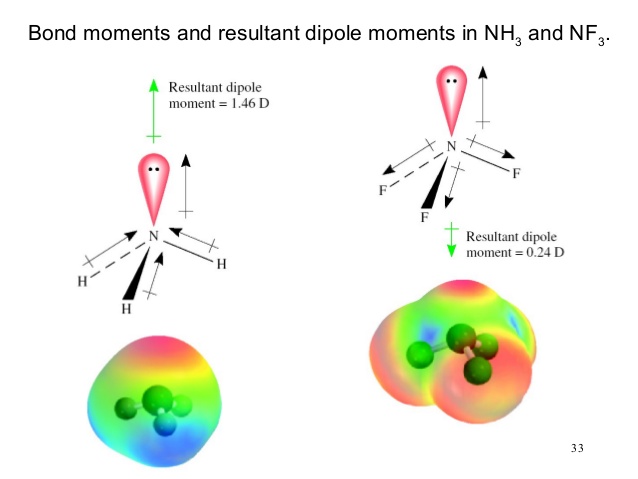
In the case of #NH_3# there are 3 hydrogen atoms bonded to the nitrogen atom, and therefore a lone pair of electrons is also present. on N. There is a pull of electron density from the H atoms towards the more electronegative nitrogen atom in the same direction as the lone electron pair, so this has the effect of boosting the dipole moment.
On the other hand, for #NF_3#, the more electronegative atom out of N and F is F. This means that the direction of pull of electron density is away from the nitrogen atom towards the 3 fluorine ones. This time the direction of pull is opposite to that of the lone pair on the nitrogen atom, and this serves to cancel out some of the dipole moment.
So #NH_3# has the higher net dipole moment.