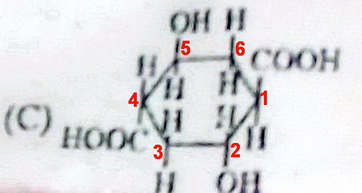
Which species exhibits a plane of symmetry?
1 Answer
Apr 29, 2018
Molecule (D) has a plane of symmetry.
Explanation:
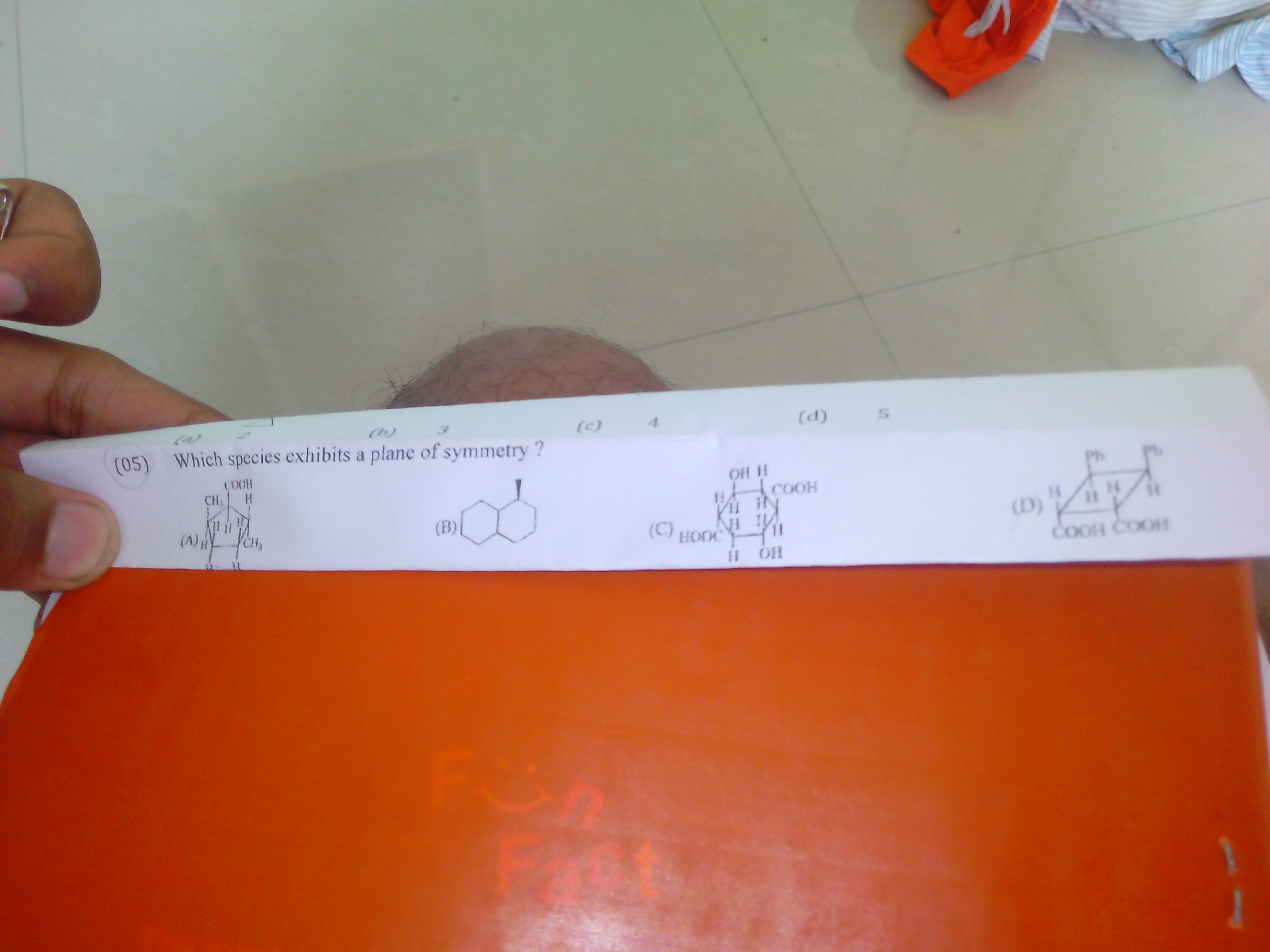
(A)
A plane of symmetry would have to be a vertical plane that includes the carboxyl group,
However, the methyl group on
Molecule (A) has no plane of symmetry..
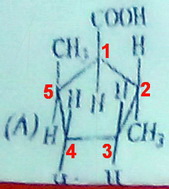
(B)
Even with a "flat" projection, Molecule (B) has no plane of symmetry.
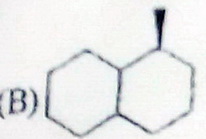
(c)
A plane of symmetry would have to include
However, the groups on
Molecule (C) has no plane of symmetry.
(D)
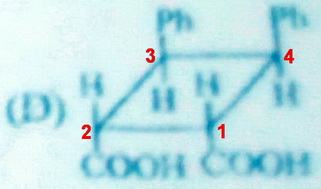
There is a vertical plane of symmetry passing through the midpoints of the
Molecule (D) has a plane of symmetry.

