Which type(s) of matter (mixtures/compounds/elements) needs to be separated by chemical methods (breaking of bonds required) such as electrolysis or decomposition?
1 Answer
Feb 1, 2017
Explanation:
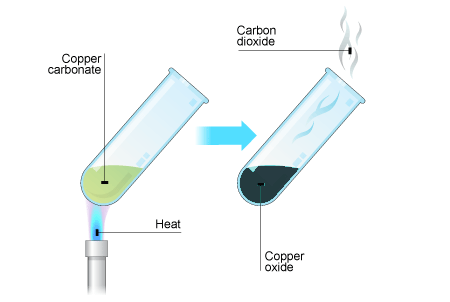
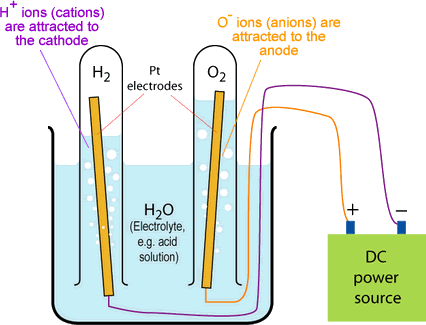
Compounds are composed of two or more elements that are chemically bonded, and can be broken down (separated by the breaking and reforming of chemical bonds) by decomposition reactions, such as electrolysis or thermal decomposition. Decomposition reactions produce individual elements or simpler compounds.
The diagram below shows the electrolysis of liquid water, producing hydrogen gas and oxygen gas.
Thermal decomposition of copper carbonate, forming copper oxide and carbon dioxide.