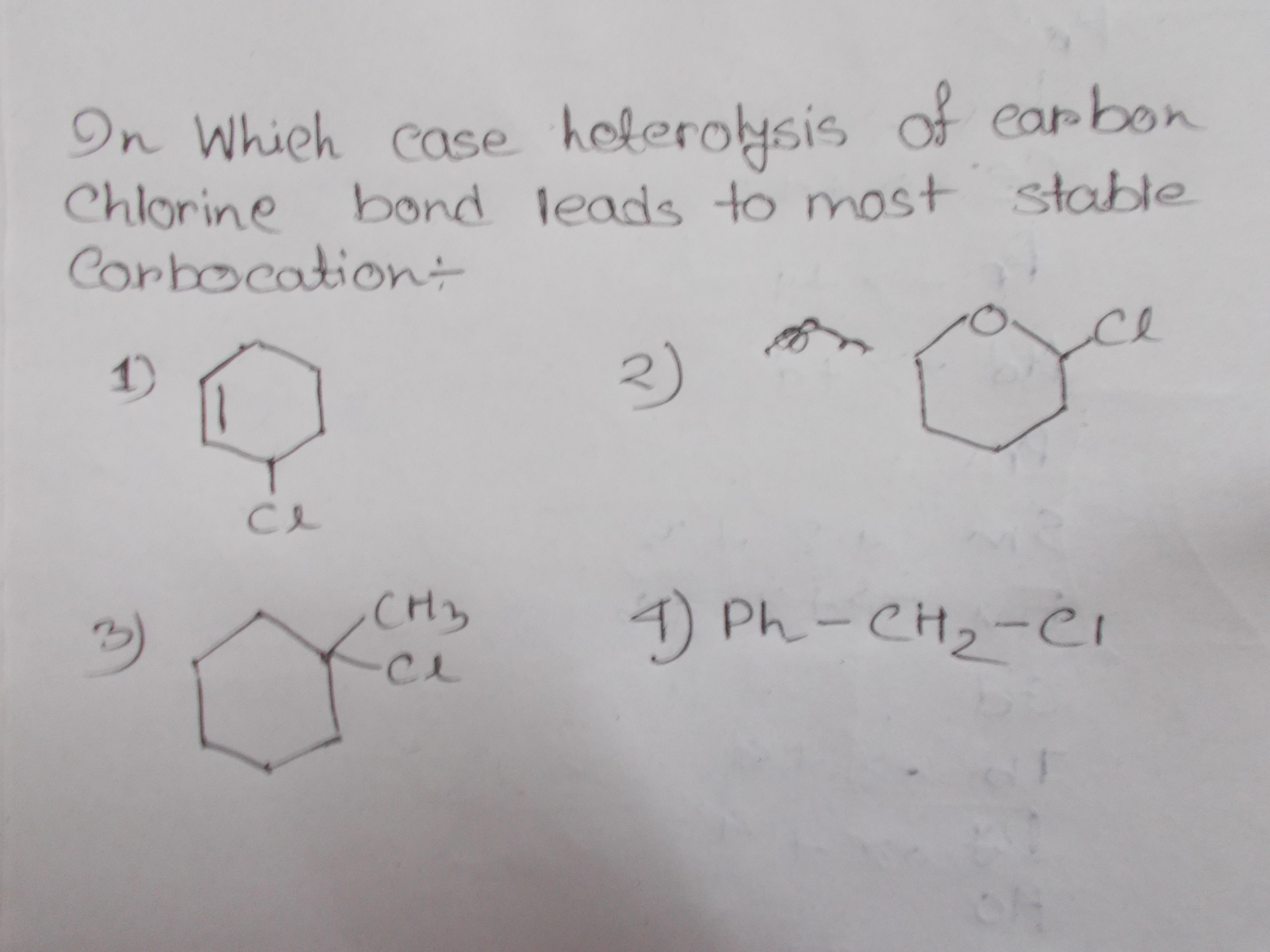
Which will be more stable carbocation upon heterolysis of #"C"-"Cl"# bond?
1 Answer
Mar 23, 2018
Consider the consequence of the heterolysis you describe for each, in turn,

This would be stabilized by resonance,


The cyclic ether would be acting as an electron donating group, stabilizing the positive charge.

This is stabilized by hyperconjugation to the highest degree (

This is a primary carbocation: a very unstable molecule.
As a result of this qualitative analysis,

