While performing the titration of a weak acid and strong base, can we put weak acid in the strong base rather than the usual strong acid in a weak base?
1 Answer
Mar 13, 2018
Yes, you could.
Explanation:
It would be unusual, but you could do it.
One problem would be that the most common indicator is phenolphthalein, which changes from colourless to pink at the endpoint.
It is easier to see the change from colourless to pink than it is to decide when the pink colour has disappeared.
However, you could use a thymol blue indicator. It changes from blue to yellow in the same pH range.
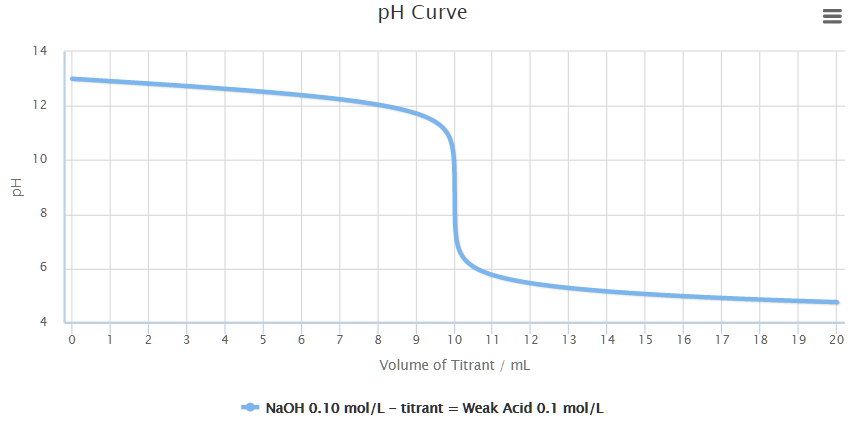
The titration curve would look something like this.